Introduction
The treatment of spasticity by the direct infusion of intrathecal baclofen (ITB), first proposed by Penn and Kroin in 1984, has met with significant success in treating patients with this condition [1]. To apply this therapy successfully, the surgeon must have a clear understanding of the definition of spasticity and the criteria with which to select appropriate patients. In a narrow physiological sense, spasticity may be defined as a motor disorder characterized by a velocity-dependent increase in muscle tone with exaggerated tendon jerks resulting from hyperexcitability of the stretch reflex [2]. The presence of spasticity can be thought of as pathognomonic of an upper motor lesion and is the result of both a facilitation and a disinhibition of the stretch reflex from a lack of input from descending cortical and spinal tracts [3]. The fact that it is defined as velocity dependent helps to distinguish it from other forms of rigidity, such as that caused by contractures, dystonias, or Parkinson’s disease. The condition of spasticity may result from many disease states originating in either the spine or the brain. Spasticity of spinal cord origin may occur with spinal cord injury, multiple sclerosis, spinal ischemia, spinal dysraphism, degenerative myelopathy, transverse myelitis, syringomyelia, spinal tumor, cervical spondylosis, and familial spastic para-paresis. Spasticity of cerebral origin may result from cerebral palsy, traumatic brain injury, cerebrovascular accident, anoxic injury, brain tumor, and metabolic diseases of the brain [4,5]. This list is by no means complete. The end result is that spasms, rigidity, and clonus interfere with the normal initiation and completion of a smooth movement and ultimately result in weakness with a loss of mobility and dexterity.
The most effective use of oral baclofen had been in the treatment of spasticity caused by spinal cord injury or multiple sclerosis. The initial in-trathecal studies were done on these patient groups after oral medications had been unsuccessful or the side effects intolerable. In these patients it was found that a small intrathecal bolus dose of baclofen was able to significantly reduce muscle tone and spasms. The effect achieved by the bolus dose could then be maintained for the long term by a continuous infusion mode. The delivery of baclofen intrathecally by a continuous infusion through a pump, in the appropriate patients, has been shown to help alleviate spasticity that may then result in improved function.
Patient Selection and Test Dosing
Baclofen, which is gamma-amino-butyric acid (GABA), acts as an agonist at the intraspinal inhibitory sites along the stretch reflex pathway, thereby effecting a decrease in the patients spasticity. Baclofen may be administered orally; however, it is water soluble and, therefore, only small amounts cross the blood-brain barrier effectively [6]. Too often maximum oral doses may not sufficiently control the patients spasticity, and patients may even experience unpleasant side effects, such as nausea, drowsiness, mental confusion, ataxia, and headache. The rationale, therefore, for administering baclofen intrathecally is that it concentrates the drug at the dorsal gray matter of the spinal cord where it is required for therapeutic effect. Furthermore, when introduced directly into the intrathecal space, effective cerebrospinal fluid (CSF) concentrations of baclofen are achieved with plasma concentrations 100 times less than those occurring with oral administration, thereby avoiding any unwanted side effects.
To assess a patient for suitability of ITB, several factors must be taken into account, including severity of spasticity, goals of the patient and family, patient responsibility to return for regular evaluations and refills, patient age, previous treatments and results, and other complicating medical conditions. Appropriate patients should be refractory to orally administered baclofen or in a situation in which their spasticity is controlled but with intolerable side effects to the drug. They should be at least 1 year posttrauma if the spasticity is of cerebral origin. A patient should initially be evaluated by the Ashworth scale (Table 1) to help quantify the severity of spasticity and for use as a baseline to determine the response to therapy. Elbow flexion and extension, wrist flexion and extension, hip abduction, knee flexion and extension, and ankle dorsiflexion are all joint functions that should be evaluated and graded on the Ashworth scale. When a patient is deemed to be an appropriate candidate, as judged by the above criteria, he or she should next undergo intrathecal test dosing.
Table 1 Ashworth Scale
|
Score |
Degree of Muscle Tone |
|
1 |
No increase in tone (normal) |
|
2 |
Slight increase in tone, giving a "catch" when affected part(s) |
|
moved in flexion or extension |
|
|
3 |
More marked increase in tone but affected part(s) easily flexed |
|
4 |
Considerable increase in tone; passive movement difficult |
|
5 |
Affected part(s) rigid in flexion or extension |
An intrathecal test dose is administered by standard lumbar puncture. Once good CSF flow is obtained through the spinal needle, a 50-microgram bolus dose is administered intrathecally. Barbitaged into the CSF over several minutes, the onset of action will generally be 30 minutes to 1 hour after administration. The peak spasmolytic effect will occur approximately 4 hours after dosing and effects may last for 4 to 8 hours. Patients are generally observed in a monitored bed setting, as adverse reactions or overdose may be life threatening. If the patient is already taking oral baclofen, this should be continued through the trial dosing, as abrupt withdrawal may result in seizures, hallucinations, and hyponatremia. If the initial dose of 50 micro-grams is ineffective in alleviating spasticity (in general, at least a 2-point reduction on the Ashworth scale is considered a good response), the test dose may be repeated after 24 hours with a 75-microgram dose. If 75 mi-crograms is again ineffective in reducing spasticity, then a third test dose may be administered 24 hours later with 100 micrograms. If there is no response after a third test dose of 100 micrograms, then the patient should be considered a nonresponder and an inappropriate candidate for ITB.
Operative Procedure
Once the preliminary test doses have established therapeutic efficacy, the patient may be scheduled for implantation of the pump. The patient must be free of any potential infectious sources, including urinary tract infections and open decubitus ulcers—conditions frequently found in this patient group.
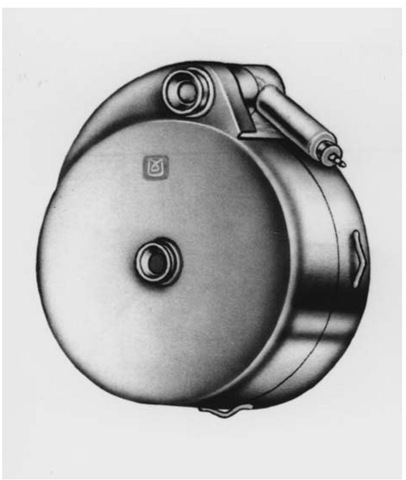
Implanting a Synchromed® Infusion System (Medtronic) is a sterile surgical procedure taking approximately 2 hours (Fig. 1). It may be performed under general, regional, or local anesthetic. The patient is typically placed in the lateral decubitus position with the right flank up. Care must be taken to pad all areas well including knees, ankles, elbows, and wrists. An axillary roll is used. The sterile field must include enough room on the anterior abdominal wall over the right upper and lower quadrants to develop a subcutaneous pocket to accommodate the pump. The field must also include the area of the lumbosacral spine at the level of L3-4.
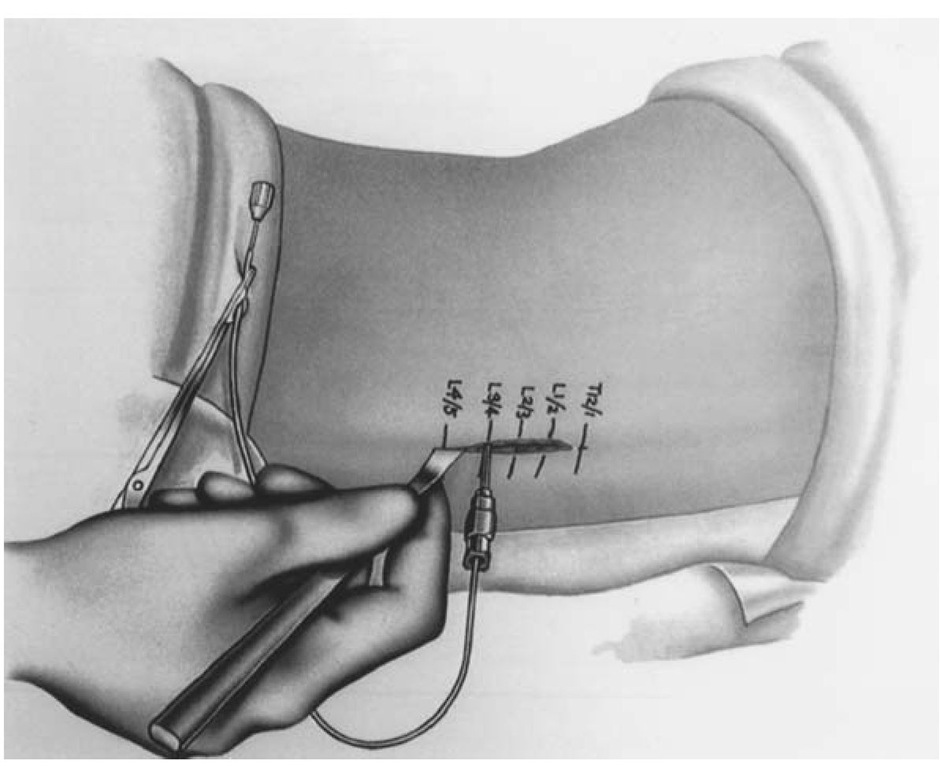
An approximately 4-cm incision should be laid out on the lumbosacral area centered over L3-4 landmarks (Fig. 2). Dissection should continue down to expose the spinous processes. With a 15-gauge Touhy needle, the thecal sac is entered. Good CSF flow is verified and sent for routine laboratory tests and cultures. The intrathecal catheter is threaded through the needle and its placement at the T10 level should be confirmed with fluo-roscopy. The Touhy needle is withdrawn and the catheter clamped to prevent additional CSF leakage.
Figure 1 Synchromed® pump.
Figure 2 Location of lumbar incision.
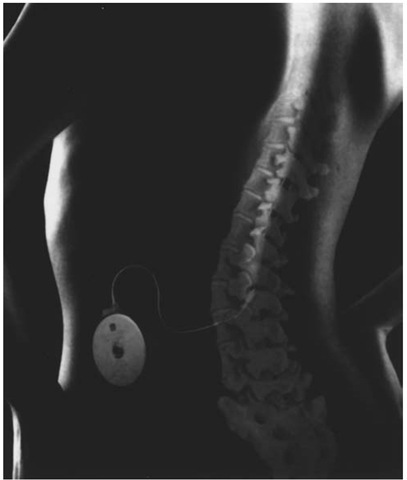
The subcutaneous pocket is developed on the anterior abdominal wall, above the rectus fascia. The pocket should be positioned sufficiently below the rib cage and above the iliac crest so that when the patient bends, the pump will not strike up against the lowest ribs or crest, causing discomfort. A larger pocket is desirable, so that the edge of the pump does not place undue pressure on the incision and precipitate an incisional dehiscence. A tunneling rod is then used to pass the proximal catheter from the pocket to the lower back incision. The catheters are then connected with the metal and Silastic connectors provided with the kit. The proximal catheter is secured to the pump, and the pump is placed within the pocket (Fig. 3). The pump may be placed in a Dacron pouch to promote fibrosis to the underlying fascia, or alternatively, newer pump series have metal loops through which the pump may be secured to the underlying fascia. One of several anchoring devices may be used to secure the distal catheter to the lumbar fascia to help prevent catheter migration. The areas are copiously irrigated with an antibiotic solution and the incisions closed in layers. Any lengths of catheter that have been trimmed away must be handed off and saved, so that the initial priming dosage may be accurately calculated. The initial dose for 24 hours is generally set at double the amount of the bolus dose shown as effective in the testing phase.
Figure 3 System in place.
The pump is then programmed with a percutaneous wand while the patient is waking up from anesthesia. The procedure for programming takes a minute or 2 to accomplish. (Refer to company manuals for exact instructions for calculating dosage rate and programming the pump.) Once the appropriate initial dosage is calculated, it should remain at this level for at least 24 hours without further changes. Thereafter, the amount delivered may be increased by 10% to 15% every 24 hours until the desired reduction in spasticity is achieved.
Postoperative Care
The patient is generally kept in the hospital for an additional 24 hours for administration of intravenous antibiotics, and a program for tapering off any oral baclofen is begun. This taper off oral medications should be accomplished gradually as the side effects of abrupt withdrawal, as mentioned above, are severe. Sutures are removed at 10 days after operation. Depending on the amount of drug required by the patient, the pump is refilled, usually by a home nursing service, every 4 to 6 weeks. Evaluation and programming of the pump function and dosage are accomplished through a percutaneous computer system on regularly scheduled patient visits. The patient should have at least a yearly evaluation by the implanting surgeon. The pump battery is expected to last between 4 to 6 years. A low battery alarm will sound with an audible beeping noise approximately 4 to 6 weeks before battery failure. Caution should be exercised in interpreting the low battery alarm, as instillation of cold medication into the pump may also trigger its sounding. If confirmed as a true battery failure, then the patient should be scheduled for a prompt revision and replacement of the pump unit. This can usually be accomplished under local anesthetic.
Complications
Infection
Infection, as can be expected with any implanted device, can be problematic. The usual signs of redness, pain, swelling and fever should be watched for closely in the immediate postoperative period. Occasionally an infection is heralded by the development of a large amount of serous fluid build-up in the subcutaneous pocket of the pump. This may be treated with aspiration of the fluid and aggressive antibiotic therapy [7]. If headache and neck stiffness develop, this may signal meningitis, and the pump system may then require removal.
Overdosage
Overdoses of intrathecal baclofen have been reported in the literature [8]. Symptoms may include drowsiness, lightheadedness, dizziness, somnolence, respiratory depression, seizures, rostral progression of hypotonia, and loss of consciousness progressing to coma. As always, emergency procedures to maintain airway, breathing, and circulation are instituted immediately. Intubation and respirator support may be required. The pump reservoir is emptied and the pump disabled. Unless contraindicated, as in patients with heart conduction defects, physostigmine is administered intravenously. In adults, 1 to 2 mg may be administered over 5 to 10 minutes. If not contraindicated, 30 to 40 ml of CSF are also withdrawn to reduce CSF baclofen concentration. The patient should respond to the physostigmine relatively rapidly but may require additional doses if the overdose was especially large. The central effects of an overdose should clear in 24 to 48 hours; however, the patient should be observed in an intensive care setting until all vital signs and mental status return to baseline.
Tolerance
Tolerance to baclofen develops in most patients over time. Over the first 6 to 12 months, the intrathecal dose may be double that of the initial dose needed to achieve the same clinical result. However, after this period the dosage will usually stabilize [9]. In a few patients, tolerance may require a drug holiday for several weeks and may be switched to intrathecal morphine, which has some of the spasmolytic effects seen with baclofen. Abrupt withdrawal of baclofen may cause seizures and hallucinations; therefore, if a patient is begun on an intrathecal drug holiday, he or she should be restarted on an oral dosage of baclofen. Furthermore, a rebound effect may be noted in which spasticity returns to an even worse level than before the start of intrathecal therapy. This effect is, however, short lived and resolves in several days.
Conclusion
Intrathecal baclofen delivered by a subcutaneously implanted programmable pump can result in a significant improvement in spasticity, as measured by Ashworth scores, and an improved quality of life for these patients.