Introduction
In the early 1980s Radionics, Inc. (Burlington, MA) was approached with a design for an innovative stereotactic frame that used computed tomography (CT) to directly target points within the brain. This device, the Brown-Roberts-Wells (BRW) frame, used a polar coordinate concept to define ste-reotactic space. A small computer with a simple menu was part of the system, thereby eliminating the need for strict orthogonality of the CT scan (a requirement of the Leksell arc-centered concept). The "picket-fence" configuration of the localizer was the key feature in this regard, as it allowed for calculation of the Z-coordinate (the height from the frame base, as opposed to the X and Y coordinates, which can be derived directly from a CT image).
The BRW frame was a great success, but further change was spurred by certain limitations. A direct approach to the posterior fossa was difficult because of the round base of the head ring. The polar coordinate system required the setting of four different angles on the arc itself, a process that could be cumbersome. A separate calculation was required for each new entry point. In 1988, introduction of the Cosman-Roberts-Wells (CRW) frame solved these problems, and the CRW design became the standard stereotactic frame made by Radionics [1].
At about the same time, a separate head ring for use with magnetic resonance imaging (MRI) scanners was introduced. Although functional, this tool was somewhat clumsy to use, and Radionics now makes one frame that is suitable for CT and MRI targeting. Application of the head ring, imaging, and use of the arc in the operating room are essentially the same as with the CRW frame, and these will be described in this topic.
Frame Application
In this era of frameless stereotaxy and emerging intraoperative imaging technologies, stereotactic frames still have their place. They are necessary for the performance of functional surgery, including placement of deep brain stimulators [2]. Brain biopsies arguably are done with the most accuracy and least time using a frame. Stereotactic radiosurgery is always done with a frame except in the unique case of the CyberKnife [3]. Craniotomies may be done in the frame, although frameless stereotaxy is much more suitable for this purpose [4].
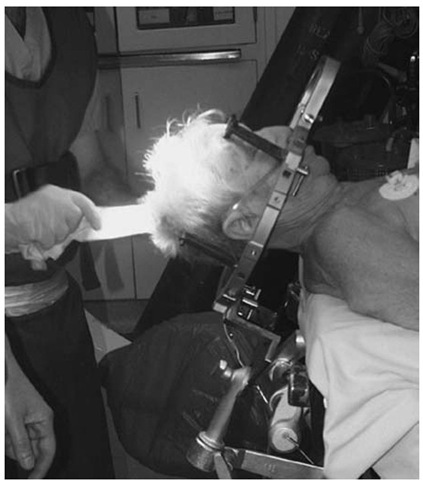
The CRW frame is applied to awake adult patients (children younger than age 12 and the rare uncooperative adult are given general anesthesia). Oral sedation (2 tablets of Percocet and 5 mg of Valium) are administered 30 minutes before the procedure. The patient remains alert with this regimen, which usually eliminates much of the discomfort of frame application. The scalp is cleaned with alcohol swabs and the patient sits up. An assistant stands behind the patient, holds the stereotactic base ring, stabilizing his hands on the patient’s shoulders. It is important to ensure that the patient’s nose will clear the ring and the overlying localizer after application is completed. The surgeon must keep the target in mind and adjust the frame location accordingly. Local anesthesia (1% lidocaine without epinephrine) is injected through the posts, and the pins are inserted until finger tight. A gentle tug on the frame checks the placement.
Imaging and Targeting
Scanning, most often with CT, is done. An adapter to the scan table, needed for radiosurgery image acquisition, is not necessary but may make scan interpretation and targeting easier. Axial scans with 3-mm slice thickness are obtained; if a lesion is known to enhance with contrast, this is given as well. Imaging for localization for functional surgery (e.g., targeting the Vim nucleus of the thalamus) should use the thinnest possible slices, usually 1 mm [5]. The scan field of view should be 34.5 to ensure inclusion of the localizer rods in the image. Gantry tilt should be avoided if a surgical navigation (SN) computer or the Radionics Stereo Calc program is to be used for image processing; if the dedicated Radionics "mini-computer" (MC) is used, then the gantry may be angled to optimize target visualization. This will be of use mainly for identifying the AC-PC line for functional targeting.
Surgery
The images are transferred to the operating room computer and the rods identified. If the MC is used, then the surgeon must enter the coordinates for each rod and for the target on the slice of interest. If need be, registration with a nonstereotactic digital image [MRI, functional MRI, position emission tomography (PET), etc.) is done. Anteroposterior, lateral, and vertical settings for the stereotactic arc are derived. In the meantime, the patient is positioned, usually supine (or lateral for an occipital or posterior fossa approach) (Fig. 1). Local anesthesia with intravenous sedation is preferred for most patients, although children will require general anesthesia.
Figure 1 Patient positioned.
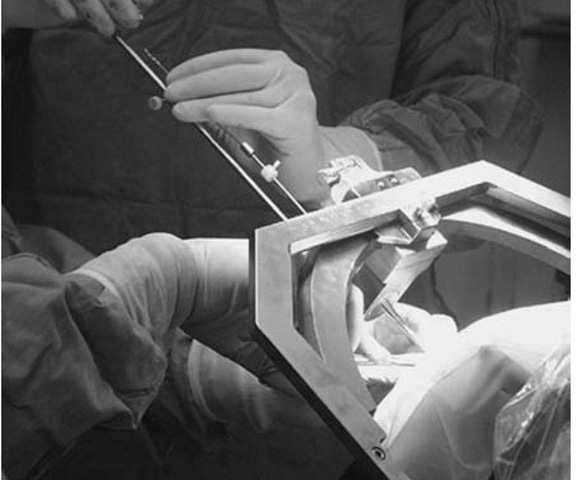
Use of the phantom base will add 5 or 10 minutes to the procedure but will give the surgeon assurance that the target will be reached using the spatial settings (Fig. 2). Sterilizing the base adds a measure of protection against infection. Coordinates are set on the phantom and arc, and a pointer is adjusted to the fixed depth from the probe holder to the target (17 cm). When the accuracy of the targeting is verified and the surgical field prepped and draped, the arc is transferred to the base ring. The arc-centered design of the CRW system allows for any entry point to be used, as long as it can be accessed through the arc and instrument holder. (Note that for temporal approaches, mounting the arc 90° to the usual orientation will give complete exposure of the area of interest).
A twist drill hole, burr hole, or craniotomy is then made, depending on the target and the surgeon’s preference. The biopsy cannula or other instrument is fixed to the appropriate length, possibly shorter or longer than the arc radius, again depending on the clinical situation. The instrument is introduced to the desired depth and the patient is examined to rule out new neurological deficits (Fig. 3). Biopsies are then taken, or other manipulations (e.g., stimulation of a functional target) are done. To move the stereotactic instrument a set depth, measure to the protruding top of the tool from a fixed object, such as the instrument holder; then with a ruler held in place, advance or withdraw the tool until the desired level is reached. Re-examine the patient after each change of instrument position. If a new deficit is noted, withdraw the instrument, close the incision, and obtain an emergency CT scan.
Figure 2 The arc on the phantom base in the operating room.
Figure 3 Insertion of a DBS electrode.
If a stereotactic biopsy is being done, frozen section confirmation that a lesional area has been targeted should be obtained; if not, specimens should be taken from different depths. If a different site needs to be targeted, this can be done quickly with an SN computer. Of course, the biopsy instrument must be withdrawn and the settings adjusted on the arc, although reconfirmation with the phantom base is not necessary. If the minicomputer was used, coordinates will need to be obtained during the surgery from the CT console—doable but cumbersome and time-consuming.
If bleeding is encountered during the procedure, irrigate patiently through the cannula. Periodically reinsert the stylet to dislodge any clot that may have formed at the cannula tip and that might falsely indicate that the hemorrhage has stopped. After the incision is closed, the patient is observed for several hours in the recovery room, a CT scan is obtained, and if no untoward findings are seen, he or she is observed in a regular hospital room overnight.
Conclusion
The CRW frame from Radionics remains a durable, versatile tool for ste-reotactic neurosurgery. Attention to detail, from frame application through imaging and the completion of surgery, will ensure a system that is safe and user friendly.