Introduction
Anterior temporal lobectomy (ATL) is the most commonly used and the most successful surgical treatment for intractable epilepsy. It is designed to treat a specific surgically remediable epilepsy syndrome: unilateral medial temporal lobe epilepsy. The specific characteristics of medial temporal lobe epilepsy were outlined in two papers from Yale University [1,2]. They include antecedent febrile convulsions in childhood, auras (abdominal/visceral, autonomic, emotional, and olfactory sensations are most common), partial complex seizures, characteristic memory deficits, and seizure onsets in the anterior temporal region. Hippocampal sclerosis is the pathological substrate of medial temporal lobe epilepsy and is present in about 80% of surgical specimens from ATL if foreign tissue lesions are excluded. It consists of a characteristic pattern of neuronal loss that is most severe in area CA1 with relative sparing of the dentate granule cells and area CA2. Axonal sprouting of the mossy fibers of the dentate granule cells into the dentate inner molecular layer is also seen. The etiology of hippocampal sclerosis and the exact relationship between the structural changes and seizure initiation remain to be completely understood. Other surgical resections for epilepsy may take place in the temporal lobe, such as foreign tissue lesionectomy or neocortical resections of the temporal cortex, but this topic will describe the standard ATL for medial temporal lobectomy as modified by Spencer [3].
Patient Selection
Surgical success is largely dependent on proper patient selection. Intractable, disabling seizures are documented by seizure diaries and trials of antiepi-leptic medications. The number of antiepileptic drugs (AEDs) for partial complex seizures is growing, but a large percentage of patients remain intractable to medical therapy. Vagus nerve stimulation should not be considered an alternative to ATL, as seizure-free rates between the two procedures are not comparable. All patients undergo history and physical examination, neuropsychological testing, video-electroencephalographic (EEG) monitoring documenting seizure onsets, and magnetic resonance (MR)-imaging with special attention to the hippocampus. All patients undergo Wada testing prior to ATL to confirm language lateralization and the adequacy of the opposite temporal lobe to support memory. Additional imaging tests may also be helpful, and these include ictal single photon emission computed tomography (SPECT), interictal positron emission tomography (PET), and magne-toencephalography. To proceed directly to ATL, we require that two major tests localize to the same temporal lobe with no discordant information from any of the other tests. In the event that temporal lobe onsets have been clearly identified but the laterality is uncertain, bitemporal depth electrodes and frontotemporal subdural strip electrodes are placed for extraoperative monitoring of ictal activity. If the seizure onsets are well lateralized but frontal cannot be clearly differentiated from temporal onsets, we will place unilateral frontotemporal subdural grid and strip electrodes.
Surgical Technique
Preoperative treatment is similar to many craniotomies. Preoperative labs are limited to a complete blood count and AED levels, unless age or other medical conditions indicate additional tests. Patients are instructed to take nothing by mouth after midnight and to take any morning AEDs when they awaken. Patients are admitted to the hospital on the day of surgery. They are given a dose of prophylactic antibiotics in the preoperative holding area. All efforts are made to maintain therapeutic AED levels throughout the surgical period. If patients are taking only oral AEDs, they are loaded with phosphenytoin intraoperatively after electrocorticography (ECoG), with the assumption that changes in gastrointestinal motility and vomiting may decrease absorption of oral medications in the early postoperative period.
At the University of Florida, a standard anesthetic regimen is given to reduce variability in intraoperative ECoG. We use a combination of isoflor-ane, nitrous oxide, and a narcotic agent at the beginning of the operation. Throughout the case, we hyperventilate (PCO2 = 30-35 mm Hg) but do not use mannitol for nonlesional cases. After the bone work is completed, iso-florane is stopped and the rest of the surgery is carried out under nitrous/ narcotic anesthesia. We do not perform awake cortical stimulation mapping when performing standard anterior temporal lobectomies.
After induction of anesthesia and intubation, the patient’s hair is clipped and shaved in the frontotemporal area. The Mayfield headholder is applied and a bump is placed beneath the contralateral shoulder. The head is turned 60 degrees from vertical with the vertex flat. Placing the vertex down is more common for craniotomies, but this angle makes the hippo-campal resection slightly more difficult. The skin incision is a typical ”question mark” that extends back to the level of the external auditory canal. The skin incision is marked, the area is sterilely prepared and draped, and the incision is made. We carry the scalp dissection full-thickness through the temporalis muscle and elevate the muscle with the scalp flap. The muscle dissection is extended so that 1 cm of the root of the zygoma in front of the ear and 1 cm of the zygomatic process of the frontal bone are exposed. The muscle and scalp flap are retracted using fish hooks and rubberbands after first wrapping the flap in a wet laparotomy pad to prevent an excessively acute angle of the scalp flap that could produce ischemic necrosis of the flap. The bone flap is made by placing burr holes above and below the pterion and over the posterior temporal lobe. The bone flap needs to provide very little frontal exposure but should be extended as low as possible over the middle fossa. Additional portions of the pterion and squamous temporal bone are removed with a Leksell ronguer. Mastoid air cells may be entered at this time and are filled with bone wax. The dura is opened in a C-shaped fashion based on the pterion. The dural flap is retracted and covered with moist gelatin sponge to prevent drying.
We perform intraoperative ECoG if no invasive monitoring has been performed previously. The results of the ECoG do not alter the boundaries of the standard resection, but we feel that it may provide additional information in the event that the patient is not seizure free after surgery. Two 4-contact strips are placed along the inferior surface of the temporal lobe. If placed correctly, the medial contacts of the strips will lie directly beneath the parahippocampal gyrus and give good recordings of medial temporal activity. A 9- or 20-contact grid is then placed over the lateral temporal lobe.
If interictal activity is extremely quiet, we will administer 20 mg of methohexital sodium to activate interictal spikes.
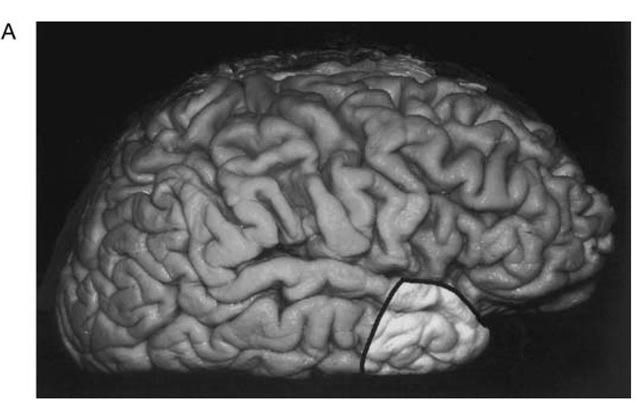
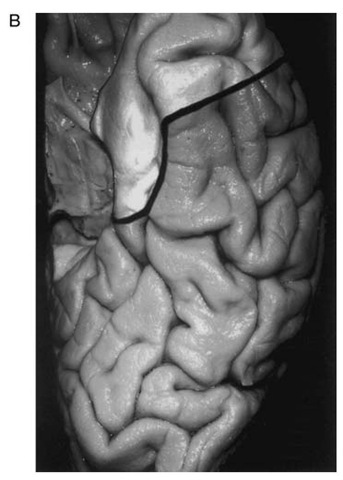
After completion of ECoG, the lateral resection margin is then measured over the middle temporal gyrus. We use 3 cm for the dominant hemisphere and 4 cm for the nondominant one (Fig. 1A). The superficial incision is then made over the lateral aspect of the temporal lobe and in the inferior aspect to the level of the collateral sulcus. We angle posteriorly as we work along the inferior surface to provide better exposure of the medial temporal structures (Fig. 1B). We then incise the pia just beneath the Sylvian fissure and coagulate and cut any anterior temporal branches of the middle cerebral artery that are confined to the area of resection. Care is taken to preserve any arteries or veins near the posterior resection margin that might be servicing tissue outside of the planned resection. The superficial Sylvian vein is also preserved as it enters the sphenoparietal sinus. The anterior portion of the superior temporal gyrus is then removed in a subpial fashion over the Sylvian fissure. Controlled, gentle suction is used so that the integrity of the pia is maintained. This dissection is continued until the lymen insulae is identified as the area of gray matter extending deep to the Sylvian fissure.
Next, the lateral cortical incision is carried out through the temporal white matter with the anterolateral temporal cortex retracted anteriorly. This dissection is extended until the temporal horn of the lateral ventricle is entered about 1 cm deep to the superior temporal sulcus. The wall of the temporal horn is heralded by the small ependymal veins running through the white matter in an anterior-posterior orientation.
Figure 1 A. Photographs of a fixed brain demonstrating the extent of cortical resection in anterior temporal lobectomy. The lateral neocortical resection extends 3 cm posterior to the tip of the temporal pole in the dominant hemisphere and 4 cm in the nondominant hemisphere.
Figure 1 B. The inferior resection angles posteriorly and the medial resection (defined laterally by the collateral sulcus) is carried back to the level of the quadrigeminal plate.
Once the temporal horn is entered, the dissection is carried anteriorly through the temporal stem to connect with the previous dissection over the Sylvian fissure. In this way the temporal horn is completely unroofed. The inferior surface of the amygdala will be appreciated forming the roof of the anterior temporal horn at this time. The anterolateral temporal specimen is then retracted laterally. The floor of the temporal horn is defined by two hemicylindrical structures running in an anterior-posterior orientation. The medial one is the dorsal aspect of the hippocampus. The lateral one is the collateral eminence. The dissection is carried through the collateral eminence to the deep surface of the collateral sulcus. The pia of the collateral sulcus is then cut, and this dissection is carried anteriorly through the rhinal sulcus. At this point, the anterolateral temporal specimen is completely separated from the brain. Any bridging veins over the surface are coagulated and cut, and the specimen is removed.
Next, the operative microscope is sterilely draped and brought into position. The Greenberg retractor system is used to retract the choroid plexus of the temporal horn superiorly and the posterior temporal cortex posteriorly. A third retractor may be used to retract the hippocampus and parahippocam-pal gyrus laterally as the dissection proceeds. The choroidal fissure is exposed and the fimbria is identified as a flap of white matter extending medially from the hippocampus. The fimbria is teased from the choroidal fissure with a microball dissector. Retracting the fimbria medially (or removing it) exposes the hippocampal sulcus. This sulcus is defined by two layers of pia with the hippocampal arteries running between them. The pial sleeves and the small arteries are coagulated and cut. This dissection is usually started over the body of the hippocampus. As the dissection is extended anteriorly, the pial sleeve will appear to extend laterally. At this point, the dissection is carried through the pes hippocampus and adjacent parahippocampal gyrus. Similarly, suction is used to transect the hippocampus and parahippocampal gyrus posteriorly. An effort is made to remove all the hippocampus except for the posterior tail. Next the dissection is carried in a subpial fashion over the subiculum and parahippocampal gyrus. When the dissection is lateral to the incisura, the pia over the parahippocampal gyrus is cut longitudinally. Anterior and middle temporal arteries from the posterior cerebral artery are coagulated and cut. The medial temporal specimen consisting of the hippocampus and parahippocampal gyrus is then removed. The lateral amygdala and the uncus are then removed subpially using gentle suction. During the medial resection, care is taken so that the integrity of the pia adjacent to the crural and ambient cisterns is preserved at all times. The resection bed is lined with a single layer of oxidized cellulose. Closure of the craniotomy is carried out in the usual fashion.
Postoperatively, patients are observed in the intensive care unit overnight. Decadron is started on the morning of surgery at 4 mg every 6 hours. On subsequent postoperative days it is reduced to 3, 2, and 1 mg every 4 hours, and then discontinued. Patients are continued on the usual AEDs. If intravenous phenytoin or phosphenytoin was initiated intraoperatively, it is continued until the patient demonstrates good oral intake. Patients are encouraged to get out of bed on postoperative day 1. Most patients return home on postoperative day 3 or 4.
Outcome
Many classification schemes have been proposed to assess outcome after surgery for intractable seizures. Perhaps the most popular is the four-tiered classification scheme developed by Engel [4]. The class 1 patients include those who are seizure-free for 2 years since surgery, those with occasional nondisabling simple partial seizures, and those with generalized convulsions only in the setting of antiepileptic medication withdrawal. Class II patients have rare (< 2-3 per year) disabling seizures. Those patients in class III have "worthwhile improvement,” generally defined as a more than 90% reduction in seizure frequency. Lastly, patients in class IV are those with no change in seizure frequency, increased seizure frequency, or a reduction in seizure frequency without an improvement in the patient’s disability. In many, the simpler scheme of seizure-free, improved, or not improved is used.
Early reports of the success of surgery in the treatment of epilepsy include those of Rasmussen in 1983 [5]. This report included 894 patients with anterior temporal lobe resections between 1928 and 1980 and documented that 37% of the patients were seizure free after 2 or more years and that 26% were improved. From the same institution, Olivier [6] more recently reported on a series of 221 patients and found that 65% were either seizure free or had a maximum of three seizures per year.
In 1989, Dasheiff [7] reviewed the available literature of reports with a minimum 2-year follow-up and found that in those reports with primarily temporal lobe resections, 40% to 62% of the patients were seizure free. An additional 21% to 35% were improved and 14% to 35% showed no improvement. In 1992, Engel [8] published the collected results of 3410 patients from 91 centers who underwent anterior temporal lobectomies and found that 67% were seizure-free, 24% were improved and 9% were not improved.
In a separate analysis, Engel [8] demonstrated the importance of following year-by-year outcomes for more than 2 years after surgery. In a group of 106 patients who underwent anterior temporal lobectomy between 1961 and 1983, only 81% of the patients who were seizure-free at 2 years remained seizure-free at 5 years, and 57% remained seizure-free at 10 years. Conversely, of those patients experiencing rare seizures after 1 year of follow-up, 57% became seizure free for at least 2 years by the end of the fifth year of follow-up. This study highlights the fact that late failures do occur and that early outcomes are not always predictive of late outcomes. Significant changes in patient outcomes continue for at least 5 to 10 years postoperatively.
Many studies have analyzed factors that may affect seizure outcomes after anterior temporal lobectomy. Dodrill et al. [9] identified patients with interictal epileptiform activity confined to the anterior temporal lobe as being likely to be seizure free. Jeong et al [10] analyzed 93 patients with mesial temporal lobe epilepsy to identify the preoperative prognostic factors that may predict good outcome after surgery. They found that 78 (84%) of the patients were seizure free at 2 years. Using multivariate analysis, they discovered that age at surgery and the presence of ipsilateral hippocampal sclerosis on MRI were the most statistically significant independent prognostic factors predictive of good outcome. This supported multiple earlier studies that identified the presence of hippocampal sclerosis, either in pathologic specimens or in preoperative MRI, as predictive of good outcome.
Foldvary et al [11] examined 79 patients who underwent anterior temporal lobectomy between 1962 and 1984. Patients were followed up for an average of 14 years, with patients having less than 2 years of follow-up being excluded from the study. Using Engel’s classification, 65% of patients were class I, 15% were class II, 11% were class III, and 9% were class IV. Using Kaplan-Meier survival analysis, it was discovered that higher preop-erative seizure frequency was associated with poor seizure outcome. In addition, seizure-free status at 2 years was found to be a better predictor of long-term outcome than the status at 6, 12, or 18 months.
Favorable outcomes after temporal lobectomy also have been correlated with the presence of abnormalities seen on PET scan [12], with nonspecific abnormalities of the mesial temporal lobe on MRI [13,14], and with functional deficits produced on intracarotid injection of amytal [15].
In patients with bitemporal abnormalities seen preoperatively on EEG, several factors may predict seizure-free status. Holmes et al [16] examined 44 patients with bitemporal, independent, interictal epileptiform patterns on EEG who underwent anterior temporal lobectomy. All patients underwent preoperative intracranial monitoring. Twenty-two (50%) of patients were seizure-free at 1 year, 14 (32%) had at least 75% reduction in seizures, and eight (18%) had less than a 75% reduction in seizures. A seizure-free outcome was associated with three independent factors: a history of febrile seizures, concordance of MRI abnormality and side of operation, and 100% lateralization of intracranially recorded ictal onset to the side of the operation.
Complication rates for ATL are about 5% in most series [17]. Possible complications that are relatively specific for ATL include verbal memory deficits and visual field defects. Removal of language-dominant medial temporal structures is associated with decreased verbal memory [18]. Most patients with dominant hippocampal sclerosis will demonstrate these deficits preoperatively (it is an integral part of the preoperative evaluation) so that few to no new deficits are seen after surgery. If a patient has normal pre-operative verbal memory, increased verbal forgetting can be demonstrated in postoperative testing. This may show a maximal effect of a loss of about 10% to 20% of preoperative ability. Patients may notice this as increased word-finding difficulties. Testing for nondominant medial temporal deficits, both pre- and postoperatively, have been relatively unrewarding, but newer, computer-based tests of visuo-spatial memory show promise in this area. Early series reported high rates of visual field defects in the contralateral superior quadrant. The incidence of this deficit has declined with ATLs that limit the lateral temporal cortical resection, and noticeable visual field defects are now rare.