1. Introduction
Modern mass spectrometry techniques such as electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) often only produce ions representing the intact molecule, usually protonated molecules (MH+). Even when the mass of these ions is measured to an accuracy that will allow for a determination of the elemental composition, the identity of the molecule’s structure is not necessarily forthcoming. Sometimes structural features of the ion representing the intact molecule can be deduced from the array of fragments formed. Both the mass of the fragments and the mass represented by the neutral loss that occurs when the ion fragments (the dark matter of the mass spectrum) are important in this process. Ions representing the intact analyte molecule produced by ESI and APCI have little or no internal energy to cause fragmentation. Therefore, it is necessary to energize these ions in order to gain the desired information.
Once formed, an ion can be made to dissociate by increasing its internal energy by any of several modes of excitation; these include collision-activated dissociation (CAD) (see Article 4, Interpreting tandem mass spectra of peptides, Volume 5) or collisionally induced dissociation (CID), surface-induced dissociation (again, involving a collision), photo-dissociation, and others. In this introduction, only CAD (same process as CID) will be considered. In CAD or CID, instrumental arrangements are available to control collisions between ions and molecules of a gas, for example, He, Ar, N2, or any other inert gas. In some cases, the ion-molecule collisions are promoted in a collision cell, which is a relatively small volume within the instrument where the gas is confined, or it can be done in a large primary segment of the mass spectrometer. The extent to which the internal energy of an ion can be increased depends on the kinetic energy of the ion and the relative masses of the ion and the gaseous molecule involved in the collision; this matter will not be addressed in this chapter.
In CAD, ions of a particular m/z value are selected (precursor ions) to collide with the collision gas molecules. Some instrumental provision is also available to determine the m/z values of the dissociation products (product ions) of these ion/molecule collisions, that is, there is one mass spectrometric means for selecting a precursor ion, and a second mass spectrometric means for analyzing the secondary ions (the product ions); thus, the overall instrumental technique is called mass spectrometry/mass spectrometry (MS/MS). The technique might consist of two separate mass spectrometers, “MS/MS-in-space”, or it might be accomplished in a fixed coordinate of space, in which case, it is called “MS/MS-in-time”; these two modes of operation will be described in detail. There are several different operational or “scan modes” used in MS/MS analyses, for example, product-ion scan, precursor-ion scan, and neutral-loss scan; these different modes of scanning will be described together with the different types of information they provide.
Mass spectrometers determine the mass-to-charge ratio (m/z) of an ion.Because mass is the principal parameter of interest, only ions of unit charge will be considered in this introductory description of MS/MS techniques. The technique of ion-ion collisions or reactions has been introduced recently as a means of reducing the charge state of multiple-charge ions; this technique will not be elaborated on here.
2. Instrumental configurations
2.1. MS/MS-in-space
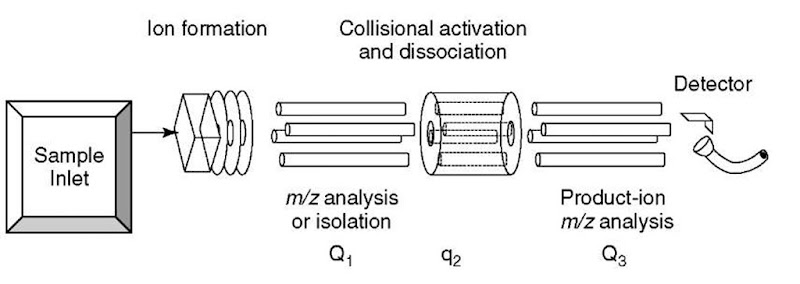
Perhaps the simplest way to visualize MS/MS is to consider two mass spectrometers connected in tandem, whereby ion current resulting from ions of a specific m/z value (or values) selected by the first mass spectrometer passes into a collision cell, where CAD occurs; ion current, resulting from fragmentation (into product ions) as well as from residual precursors ions, passes out of the collision cell into the second mass spectrometer, where it is analyzed according to individual m/z values as recorded by a detector at the end of the second instrument. The triple-quadrupole mass spectrometer illustrated in Figure 1 is a manifestation of this MS/MS-in-space technology. The ions are selected in the first quadrupole (Q1) mass spectrometer (this instrument is not scanned), then passed to the second quadrupole (q2), which is not mass-selective, that is, it is not a mass spectrometer, but rather it provides a means to “refocus” scattered ions in the collision cell where some of the precursor ions are transformed by CAD into product ions; all the ions leave the collision cell and enter the third quadrupole (Q3) mass spectrometer where they are m/z-analyzed. These instruments are called triple quadrupoles because the originally developed concept used three quadrupole devices (see Article 8, Quadrupole mass analyzers: theoretical and practical considerations, Volume 5). In modern instruments that still use a quadrupole m/z analyzer for Qi and Q3, the device constituting q2, the collision cell, is something other than a quadrupole; it is usually a hexapole or octupole device or, as in the case of more recent instrumentation, a lens stack.
Figure 1 Schematic diagram of a triple-quadrupole mass spectrometer
2.2. MS/MS-in-time
An increasingly common technique is to perform selection, dissociation, and analysis of secondary ions as a function of time in the same coordinates of space within a mass spectrometer. The quadrupole ion trap (QIT) (see Article 9, Quadrupole ion traps and a new era of evolution, Volume 5) mass spectrometer has become a popular instrument for conducting MS/MS-in-time; the ion cyclotron resonance mass spectrometer can also be used for this purpose, using technology commonly called FTMS (see Article 5, FT-ICR, Volume 5). An important limitation of MS/MS-in-time is referred to as the “low-mass cutoff”. Product ions that have an m/z value of less than approximately one-third that of the precursor ion cannot be stored while CAD of the precursor ion is being carried out. An advantage of MS/MS-in-time over MS/MS-in-space is that only ions with the m/z value of the precursor ion are collisionally activated, meaning that secondary fragmentation is less likely to take place than in the collision cell of the MS/MS-in-space configuration, where product ions are also likely to undergo collisional activation and secondary dissociation.
3. Different “ion-scanning modes” for MS/MS with CAD
3.1. Product-ion scan
Probably the most commonly employed technique for MS/MS is one in which a particular precursor ion is subjected to CAD to obtain a product-ion mass spectrum to serve as a “fingerprint”; this MS/MS-in-space mode of operation is carried out essentially as described above. The MS/MS-in-time mode of operation in a quadrupole ion trap begins by transferring all the ions generated by a given sample into the trap, then adjusting the rf/dc parameters of the instrument to eject ions of all m/z values except those of the precursor. The normal operating pressure of the QIT is about the same as that of the collision cell in the triple quadrupole (0.13 Pa); therefore, if ions corresponding to the m/z value of the precursor ion are then excited, they will undergo collisions with the gas molecules in the trap and dissociate. The second step of the analysis begins by adjusting the rf/dc parameters of the instrument to accommodate ions through a large range of m/z values even though the precursor ions are the only ones in the trap; in this way, any nascent product ions produced during CAD of the precursor ions will be trapped as they are produced. The third step consists of a mass-selective ejection scan of the rf/dc parameters to obtain a mass spectrum of the product ions.
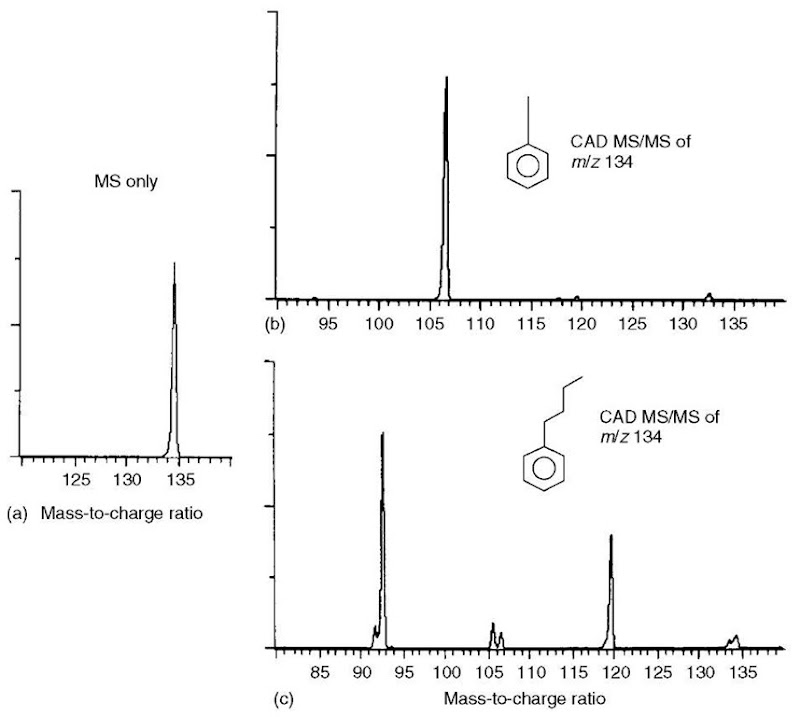
Figure 2 (a) Conventional mass spectrum (no CAD) of either t-butyl benzene or n-butyl benzene (each has a molecular ion peak at m/z 134 and no significant fragment ion peaks). (b) and (c) CAD MS/MS spectra of ion current at m/z 134 from t-butyl benzene and n-butyl benzene, respectively
The power of the product-ion scan is demonstrated in a comparison of Figure 2(b) and (c), each of which is a product-ion spectrum of a precursor ion of m/z 134. Clearly, the pattern of peaks in the two product-ion mass spectra is different. In this way, the product-ion spectrum serves as a fingerprint for each of two compounds, each of which is capable of generating a precursor ion of m/z 134. Obviously, the ion of m/z 134 has a different structure in each case, which is the basis for the CAD product-ion spectra being different.
3.2. Precursor-ion scan
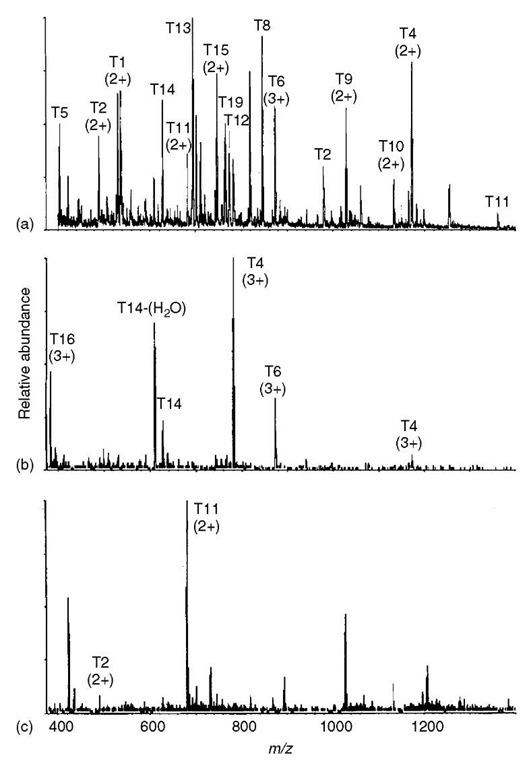
In this scan mode, the second mass spectrometer in the MS/MS-in-space configuration is held at a constant parameter (not scanned), while the first mass spectrometer is scanned through a specified range of m/z values. In this way, an array of precursor ions can be assessed for their capacity to generate a product ion of a specific m/z value. An application of a precursor-ion scan is illustrated with the spectrum in Figure 3(b). From previous work, it was known that CAD of protonated peptides containing tyrosine produces the immonium ion of tyrosine at m/z 136, that is, the generation of such a product ion can be used as a marker for the presence of tyro-sine in a peptide. In this application, the tryptic digest was analyzed by ESI CAD MS/MS by scanning the first mass spectrometer, while holding the second mass spectrometer constant at m/z 136; in this mode of operation, the detector produces a signal at a given m/z value only when the corresponding precursor ion in the scan of the first mass spectrometer is capable of producing an ion of m/z 136 in the collision cell. The complexity of the mixture in this application is represented in Figure 3(a), which is a mass spectrum obtained from analysis of the digest by direct infusion ESI-MS. Notice that the spectrum obtained during ESI-MS/MS with precursor scanning for m/z 136 (Figure 3b) is much simpler indicating that only four of the tryptic peptides (T4-T6, T14, and T16) contain tyrosine.
3.3. Neutral-loss scan
Under CAD conditions, some precursor ions are capable of expelling a molecule (the neutral dark matter), such as CO2, methanol, and so on, in forming the product ion (see Article 4, Interpreting tandem mass spectra of peptides, Volume 5). This phenomenon of neutral loss can be exploited in detecting members of a family of such compounds during the analysis of a complex mixture by CAD MS/MS using the neutral-loss scan. The neutral-loss scan is accomplished by arranging a simultaneous scan of both mass spectrometers (one before, the other following the collision cell, where CAD takes place) offset from one another by a difference in m/z values equal to the mass of the molecule expelled from the precursor ions of interest. An application of a neutral-loss scan is illustrated in Figure 3(c). From previous work, it was known that double-protonated peptides containing methionine eliminated CH3SH (48 Da) during CAD. The spectrum in Figure 3(c) indicates that only two of the tryptic peptides, namely, T2 and T11, among the 20 or so peptides represented in the top spectrum from nonspecific analysis of the digest contain methionine; the data for Figure 3(c) were recorded by scanning Q1 and Q3 offset by 24 m/z units (mass of 48 Da divided by charge of 2).
It is important to note that the precursor-ion scan and the neutral-loss scan can only be carried out using MS/MS-in-space. These two modes of operation are not possible with MS/MS-in-time. When one of the m/z analyzers in an MS/MS-in-space instrument is a nonscanning mass spectrometer, such as a time-of-flight (see Article 10, Hybrid MS, Volume 5) m/z analyzer, the neutral-loss scan mode is not possible because the second analyzer does not scan and, therefore, cannot have a scan offset.
Another important mode of operation for MS/MS is the selected reaction monitoring (SRM) mode. This mode is used for quantitation and specificity. Q1 is held at a fixed value to allow precursor ions of the specified m/z value to pass into the collision cell (q2). Q3 is held at a fixed m/z value to allow only product ions of a specific mass to reach the detector. In this way, specificity is achieved because only ions that undergo a transition from m/z A to m/z B in the collision cell will reach the detector; peak area integrated from the product-ion signal provides quantitative information.
Figure 3 Two examples of results from specialized uses of CAD MS/MS spectra. (a) Conventional electrospray mass spectrum (MS only, no CAD MS/MS) resulting from infusion (no chromatography) of a mixture of tryptic peptides into an electrospray ionization triple-quadrupole mass spectrometer; numbers in parentheses indicate charge state of the corresponding ion. (b) Precusor-ion mass spectrum of the same mixture acquired under conditions allowing detection of only those precursors capable of generating a product ion of m/z 136 (immonium ion of tyrosine = 136 Da). (c) Neutral-loss mass spectrum of the same mixture acquired at an offset in m/z value between Q1 and Q3 to allow detection of only those precursors capable of expelling CH3SH (48 Da) indicating the presence of methionine in the side chain.
4. Summary
CAD can provide characteristic, if not unique, fragmentation (into product ions) of a precursor ion. A variety of scan modes is available with MS/MS instrumentation to permit selective detection of certain compounds depending on the CAD behavior of the precursor ions.