1. Introduction
Mass spectrometry (MS) is an essential core technology in proteomics, the comprehensive analysis of the proteins expressed in a cell or tissue (Aebersold and Goodlett, 2001). In the late 1980s, two new ionization methods were developed that have revolutionized the analysis of large biomolecules by MS.
Both methods, electrospray ionization (ESI, Fenn etal., 1989) and matrix-assisted laser desorption/ionization (MALDI, Karas and Hillenkamp, 1995), convert nonvolatile analytes like proteins and peptides to ions in gas phase without derivatization and minimal fragmentation. These ions are then selected to mass analysis. Because of the lack or minimal extent of analyte fragmentation during ionization, ESI and MALDI are also referred to as soft ionization methods (Aebersold, 2003). ESI generates ions by spraying a solution at atmospheric pressure through a small-diameter needle and by applying electric potential between the needle and the inlet of a mass spectrometer. Through evaporation and splitting, the size of the droplets is reduced. This desolvation process ultimately produces bare analyte ions in the gas phase, which are then transmitted to the mass spectrometer. Because of its solution-based nature, ESI is easily coupled on line with liquid phase separation techniques, particularly with high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE). Furthermore, due to the propensity of ESI to produce multiple charged ions, simple quadrupole instruments and other types of mass analyzers with limited mass/charge (m/z) range could be used to detect ions with masses exceeding the nominal m/z range of the instrument (typically 0-4000 Da). For MALDI, analytes are cocrystallized with a UV-absorbing compound (matrix) on a sample probe. Pulses of UV laser light are used to vaporize small amounts of the matrix and analyte ions to produce protonated gas phase ions. The method is relatively resistant to contaminating species and, due to the propensity to generate predominantly singly charged ions, MALDI spectra are simple to explain (Aebersold and Goodlett, 2001).
Over the last decade increasingly sophisticated and powerful mass spectrometers for peptide analysis have been developed and interfaced with high-performance separation systems. Separations prior to MS analysis remove salts and other impurities and concentrate analytes in the narrow elution peaks, resulting in increased sensitivity. This review will focus on the recent advances of on-line high-performance separation techniques such as HPLC and capillary zone electrophoresis (CZE) for MS analysis in proteomics.
2. Reversed-phase microcapillary chromatography and ESI-MS
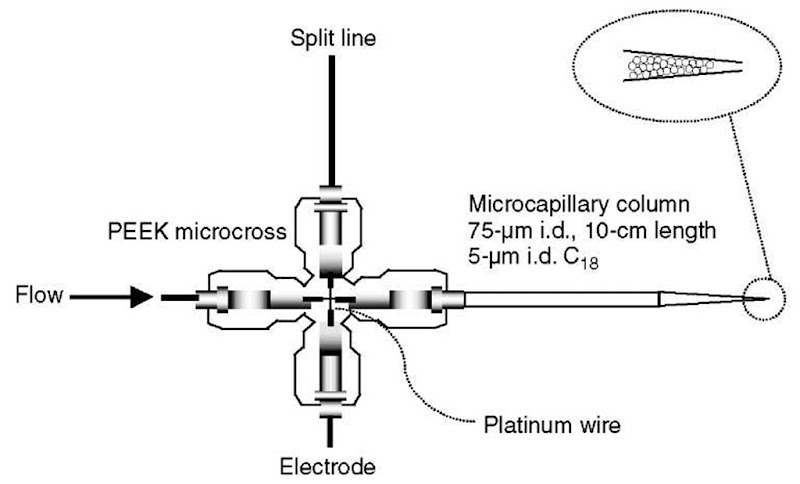
Nanospray ionization, a low flowrate ESI, gained wide popularity due to its high sensitivity. Wilm and Mann proposed that the desorption efficiency of analyte peptide ions from the electrosprayed droplet increases as the size of the droplets decreases. This is thought to be due to the larger surface area of the droplet in relation to its total volume (Wilm and Mann, 1996). As a result, a greater proportion of the available analyte molecules is ionized and transmitted to mass spectrometers. This enhanced sensitivity of the nanospray method reduced the amount of peptide required for MS analysis to a few femtomoles and below in the mid-1990s (Shevchenko et al., 1996). Efforts to miniaturize HPLC to utilize nanospray ionization in combination with on-line peptide separation lead to the development of packed microcolumns using fused silica capillaries with a 20-150-|m inner diameter (i.d.) and a flowrate of less than 1 |l min-1 (Ishihama, 2005; Moseley et al., 1991; Holland and Jorgenson, 1995; Emmett and Caprioli, 1994). Even though the sensitivity of MS analysis increases with decreasing column i.d., most microcapillary liquid chromatography (|LC) work is done with 75 or 100 |m i.d. capillary columns because these clog less frequently than capillary columns of <50 |m i.d. Using this microscale format, reversed-phase liquid chromatography has most often been combined with mass spectrometric analysis of a protein digest because the on-line separation also effectively removes contaminants such as salts and detergents that are detrimental to ESI performance. Figure 1 shows an integrated |LC/nanospray ionization device (Gatlin et al., 1998). The microcross works as a flow splitter providing nanoliter per minute scaled flow to the column and as a junction point of liquid and the metal wire supplying the electric potential required for nanospray ionization. One advantage of this type of device is the use of the capillary tip, tapered to ~5 |m, to hold the Ci8-derivatized particles in place rather than use of a sintered frit connected to a separate ESI emitter via a union. This design significantly reduces the dead volume and band broadening. This type of integrated device has gained popularity from work by Yates and coworkers (Gatlin et al., 1998), most notably as multidimensional protein identification technology (MudPIT) using a biphasic capillary column (Link et al., 1999).
The integrated device has been further developed by several groups (Yi et al., 2003; Meiring et al., 2002; Licklider et al., 2002). The main advantage is the introduction of a sample trapping and enriching precolumn to enable automated consecutive runs for high-throughput analysis. The precolumn allows decreased sample loading time, rapid sample desalting, and facilitates automation. Despite these advantages, the dead volume between the precolumn and the microcapillary analytical column can be a limiting factor. The consequent band broadening will decrease ESI sensitivity for those analytes present at low relative stoichiometry. To minimize the dead volume, a precolumn and a microcapillary column were integrated as one piece (Meiring etal., 2002; Licklider etal., 2002), or the two columns were placed in close proximity (Yi etal., 2003). The ruggedness and sensitivity of such devices was demonstrated by performing more than 60 consecutive |LC separations on complex peptide mixtures without degradation of chromatographic resolution and by detecting 1 amol of a standard peptide using a conventional ion-trap mass spectrometer (Yi etal., 2003).
Figure 1 Schematic representation of |LC/ESI device
Further increases of sensitivity into the zeptomole (10-21 moles) range was achieved by combining ultra high-pressure |LC separating at a pressure of 10000psi with Fourier transform ion-cyclotron resonance (FTICR) mass spectrometer (Shen et al., 2003; Belov et al., 2004).
Recently, Yin etal. (2005) have developed a novel integrated nano-LC/ESI microfluidic chip in which an enrichment column, a reversed-phase column (75-|m width x 50-|m depth x 40-mm length), and an ESI tip have been laser-ablated into a polyimide film. The compact format of the chip reduces the number of capillary tubing and fittings required and greatly simplify operation skills. It also facilitates troubleshooting for blockages and leakages in capillaries and connectors. Initial efforts using this chip coupled to ion-trap mass spectrometer have resulted in the identification of 47 protein clusters from albumin and IgG-depleted rat plasma sample by the chip separation alone, and 111 clusters by two-dimensional separation of strong cation exchange (SCX) chromatography and the chip (Fortier et al., 2005).
3. Capillary zone electrophoresis and ESI-MS
For CZE or simply CE, separations occur inside a capillary filled with a moderately conductive liquid. The degree of separation of analytes inside the capillary depends on their relative electrophoretic mobilities under the applied electrical field. CE has been widely utilized for separations of proteins and peptides due to the high separation efficiency of the method that reaches ~106 theoretical plates, and high speed (Shen and Smith, 2002; Simpson and Smith, 2005). A critical disadvantage of CE, however, is the limited sample injection volume, typically below 20 nl (~1% of the total capillary column volume) (Shen et al., 2000), which limits the sample concentration and limits of detection. To overcome this limitation, various preconcentration methods have been implemented including sample stacking, transient isotachophoresis, and solid-phase extraction (Stroink et al., 2001). Among the various preconcentration methods, solid-phase extraction has been effectively coupled with CE for on-line interfacing with mass spectrometers. Figeys and coworkers used a microfluidic device integrating a C18-based preconcentrator and CE for the analysis of protein digests (Figeys et al., 1999; Figeys et al., 1998).
ESI is the predominant method for interfacing CE to MS, while MALDI has also been used extensively. ESI-based interfaces divide into two categories: sheath-flow interfaces and sheathless interfaces (Schmitt-Kopplin and Frommberger, 2003; Moini, 2002). For the sheath-flow interface, a second tube of a larger diameter surrounds the separation capillary in a coaxial arrangement. The sheath liquid is driven through the outer tube by an external pump. The sheath-flow interfaces provide system stability and reduce the requirement for the background electrolyte to be compatible with ESI-MS. These advantages make those most widely used interface designs (Simpson and Smith, 2005). The main disadvantage of the sheath-flow configuration, however, is low detection sensitivity due to the dilution of the analyte by the sheath liquid (Moini, 2002). Sheathless interfaces offer higher sensitivity because there is no sheath liquid present to dilute the CE effluent. A variety of the sheathless interfaces have been reviewed by Issaq et al. (2004).
4. Conclusions
Major analytical challenges in proteomics are the enormous complexity of the proteome and the large variance in protein abundances. Thus, with advances in MS technology, the initial efforts of proteomics have focused on improving interfaces between the separation techniques and MS to increase detection sensitivity. Concurrently, another effort to combine other separation techniques, such as SCX, affinity chromatography, free-flow electrophoresis, capillary isoelectric focusing, and one-dimensional electrophoresis, with liquid chromatography – mass spectrometry (LC-MS) and CE-MS have been carried out to enhance the resolution and dynamic range of proteome-wide analysis (Han et al., 2001; Moritz et al., 2004; Chen et al., 2003; Aebersold and Goodlett, 2001). It can be expected that with these developments complete proteome analysis will become a reality.