OPERATION OF THE FISHER-JOHNS APPARATUS
1. Don’t assume that the unit is cold. That is a good way to get burned.
2. Keep your grubby fingers off the cover slides. Use tweezers or forceps.
3. Place a clean round glass cover slide in the well on the hot stage. Never melt any samples directly on the metal stage. Ever!
4. Put a few crystals on the glass. Not too many. As long as you can see them melt, you’re all right.
5. Put another cover slide on top of the crystals to make a sandwich.
6. Set the voltage control to zero if it’s not already there.
7. Turn on-off switch to ON. The light source should illuminate the sample. If not, call for help!
8. Now science turns into art. Set the voltage control to any convenient setting. The point is to get up to within 20° C of the supposed melting point. Yep, that’s right. If you have no idea what the melting point is, it may require several runs as you keep skipping past the point with a temperature rise of 5 -10° C per minute. A convenient setting is 40. This is just a suggestion, not an article of faith.
9. After you’ve melted a sample, let it cool, and remove the sandwich of sample and cover slides. Throw it away! Use an appropriate waste container.
10. Once you have an idea of the melting point (or looked it up in a handbook, or you were told), get a fresh sample, and bring the temperature up quickly at about 5-10°Cper minute to within 20 ° C of this approximate melting point. Then turn down the voltage control to get a 2°C per minute rise. Patience!
11. When the first crystals just start to melt, record the temperature. When the last crystal just disappears, record the temperature. If both points appear to be the same, either the sample is extremely pure, or the temperature rise was too fast.
12. Turn the on-off switch to OFF. Now set the voltage control to zero.
13. Let the stage cool, then remove the sandwich.
THE THOMAS-HOOVER APPARATUS
The Thomas-Hoover apparatus (Fig. 36) is the electromechanical equivalent of the Thiele tube or open beaker and hot oil methods (see "Using the Thiele Tube"). It has lots of features, and you should look for the following.
Fig. 36 The Thomas-Hoover apparatus.
1. Light box. At the top of the device, towards the back, a box holds a fluorescent light bulb behind the thermometer. On the right side of this box are the fluorescent light switches.
2. Fluorescent light switches. Two buttons. Press and hold the red button down for a bit to light the lamp; press the black button to turn the lamp off.
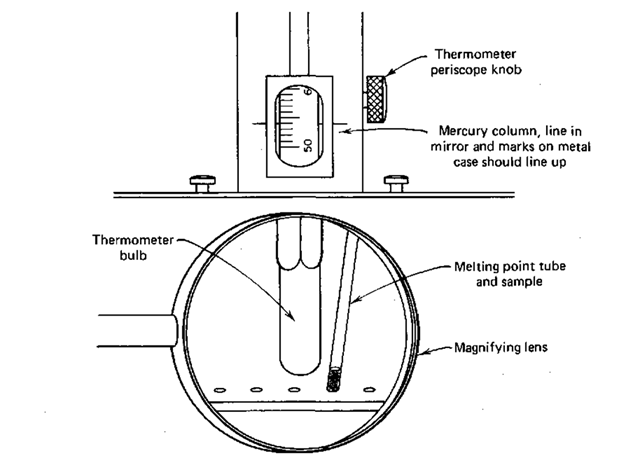
3. Thermometer. A special 300° thermometer in a metal jacket is immersed in the oil bath that’s in the lower part of the apparatus. Two slots have been cut in the jacket to let light illuminate the thermometer scale from behind, and to let a thermometer periscope read the thermometer scale from the front.
4. Thermometer periscope. In front of the thermometer, this periscope lets you read a small magnified section of the thermometer scale. By turning the small knob at the lower right of this assembly, you track the movement of the mercury thread, and an image of the thread and temperature scale appear in a stationary mirror just above the sample viewing area.
5. Sample viewing area. A circular opening cut in the front of the metal case such that you can see your samples in their capillary tubes (and the thermometer bulb) all bathed in the oil bath. You put the tubes into the oil bath through the holes in the capillary tube stage.
6. Capillary tube stage. In a semicircle about the bottom of the jacketed thermometer, yet behind the thermometer periscope, are five holes through which you can put your melting point capillaries.
7. Heat. Controls the rate of heating, not the temperature. The higher the setting, the faster the temperature rise. At Hudson Valley Community College, we’ve had a stop put in and you can only turn the dial as far as the number 7. When it gets up to 10, you always smoke the oil. Don’t do that.
8. Power on – off switch. Turns the unit on or off.
9. Stirrer control. Sets the speed of the stirrer from low to high.
10. Vibrator on-off switch. Turns the vibrator on or off. It’s a spring-return switch so you must hold the switch in the on position. Let go, and it snaps off.
11. Line cords. One brings a.c. power to the heater, stirrer, sample light, and vibrator. The other cord brings power to the fluorescent light behind the thermometer. Be sure both cords are plugged into live wall sockets.
OPERATION OF THE THOMAS-HOOVER APPARATUS
1. If the fluorescent light for the thermometer is not lit, press the red button at the right side of the light box and hold it down for a bit to start the lamp. The lamp should remain lit after you release the button.
Fig. 37 Close up of the viewing system.
2. Look in the thermometer periscope, turn the small knob at the lower right of the periscope base, and adjust the periscope to find the top of the mercury thread in the thermometer. Read the temperature. Wait for the oil bath to cool if the temperature is fewer than 20 Celsius degrees below the approximate melting point of your compound. You’ll have to wait for a room temperature reading if you have no idea what the melting point is. You don’t want to plunge your sample into oil that is so hot it might melt too quickly, or at an incorrect temperature.
3. Turn the voltage control to zero if it isn’t there already.
4. Turn the power on-off switch to ON. The oil bath should become illuminated.
5. Insert your capillary tube in one of the capillary tube openings in the capillary tube stage. This is not simple. Be careful. If you snap a tube at this point, the entire unit may have to be taken apart to remove the pieces. It appears you have to angle the tube toward the center opening and angle the tube toward you (as you face the instrument) at the same time (Fig. 37). It’s as if they were placed on the surface of a conical funnel.
6. Adjust the magnifying glass for the best view of your sample.
7. Turn the stirrer knob so that the mark on the knob is about half of the way between the SLOW and FAST markings on the front panel. That’s just a suggestion. I don’t have any compelling reasons for it.
8. Adjust the thermometer periscope to give you a good view of the top of the mercury thread in the thermometer.
9. Now science turns into art. Set the heat control to any convenient setting. The point is to get up to within 20°C of the supposed melting point. If you he e no idea what the melting point is, it may require several runs as you keep skipping past the point with a temperature rise of 5-10° C per minute. A convenient setting is 4. This is just a suggestion, not an article of faith.
10. Remember, you’ll have to keep adjusting the thermometer periscope to keep the top of the mercury thread centered in the image.
11. After you’ve melted a sample, throw it away!
12. Once you have an idea of the melting point (or looked it up in a handbook, or were told), get a fresh sample, and bring the temperature up quickly at about 5-10°C per minute to within 20° C of this approximate melting point. Then turn down the heat control to get a 2° Cper minute rise. Patience!
13. When the first crystals just start to melt, record the temperature. When the last crystal just disappears, record the temperature. If both points appear to be the same, either the sample is extremely pure, or the temperature rise was too fast. If you record the temperature with the horizontal index line in the mirror matched to the lines etched on both sides of the periscope window and the top of the mercury thread at the same time, you’ll be looking at the thermometer scale head on. This will give you the smallest error in reading the temperature (Fig. 38).
14. Don’t turn the control much past 7. You can get a bit beyond 250°C at that setting, and that should be plenty for any solid compound you might prepare in this lab. Above this setting, there’s a real danger of smoking the oil.
15. Turn the power switch to OFF. You can also set the heat control to zero for the next person.
16. Press the black button on the right side of the light box and turn the fluorescent light off.
17. Remove all capillary tubes.
Fig. 38 Reading the temperature.
There are a few more electric melting point apparatus around, and much of them work the same. A sample holder, magnifying eyepiece, and voltage control are common, and an apparently essential feature of these devices is that dial markings are almost never temperature settings. That is, a setting of 60 will not give a temperature of 60 °C, but probably much higher.
USING THE THIELE TUBE
With the Thiele tube (Fig. 39) you use hot oil to transfer heat evenly to your sample in a melting point capillary, just like the metal block of the Mel-Temp apparatus does. You heat the oil in the sidearm and it expands. The hot oil goes up the sidearm, warming your sample and thermometer as it touches them. Now, the oil is cooler and it falls to the bottom of the tube where it is heated again by a burner. This cycle goes on automatically as you do the melting point experiment in the Thiele tube.
Fig. 39 Taking melting points with the Thiele tube.
Don’t get any water in the tube or when you heat the tube the water can boil and throw hot oil out at you. Let’s start from the beginning.
Cleaning the Tube
This is a bit tricky, so don’t do it unless your instructor says so. Also, check with your instructor before you put fresh oil in the tube.
1. Pour the old oil out into an appropriate container and let the tube drain.
2. Use a hydrocarbon solvent (hexane, ligroin, petroleum ether—and no flames!) to dissolve the oil that’s left.
3. Get out the old soap and water and elbow grease, clean the tube, and rinse it out really well.
4. Dry the tube in a drying oven (usually > 100° C) thoroughly. Carefully take it out of the oven and let it cool.
5. Let your instructor examine the tube. If you get the OK, then add some fresh oil. Watch it. First, no water. Second, don’t overfill the tube. Normally, the oil expands as you heat the tube. If you’ve overfilled the tube, oil will crawl out and get you.
Getting the Sample Ready
Here you use a loaded melting point capillary tube (see "Loading the Melting Point Tube") and attach it directly to the thermometer. The thermometer, unfortunately, has bulges; there are some problems, and you may snap the tube while attaching it to the thermometer.
1. Get, or cut, a thin rubber ring from a piece of rubber tubing.
2. Put the bottom of the loaded M.P. tube just above the place where the thermometer constricts (Fig. 40), and carefully roll the rubber ring onto the M.P. tube.
3. Reposition the tube so that the sample, is near the center of the bulb and the rubber ring is near the open end. Make sure the tube is vertical.
Dunking the Melting Point Tube
There are more ways of keeping the thermometer suspended in the oil than I care to list. You can cut or file a notch On the side of the cork, drill a hole, and insert the thermometer (Be careful!) Finally, cap the Thiele tube (Fig. 39). The notch is there so that pressure will not build up as the tube is heated. Keep the notch open, or the setup may explode.
Fig. 40 Attaching M. P. tube to thermometer without a disaster.
But this requires drilling or boring corks, something you try to avoid (why have ground glass jointware in the undergraduate lab?). You can gently hold a thermometer and a cork in a clamp (Fig. 41). Not too much pressure, though!
Finally, you might put the thermometer in the thermometer adapter and suspend that, clamped gently by the rubber part of the adapter, not by the ground glass end. Clamping ground glass will score the joint.
Heating the Sample
The appropriately clamped thermometer is set up in the Thiele tube as in (Fig. 39). Look at this figure now and remember to heat the tube carefully— always carefully — at the elbow. Then:
1. If you don’t know the melting point of the sample, heat the oil fairly quickly, but no more than 10° Cper minute to get a rough melting point.
Fig. 41 Safely suspended thermometer with Thiele tube.
And it will be rough indeed, since the temperature of the thermometer usually lags that of the sample. 2. After this sample has melted, lift the thermometer and attached sample tube carefully (it may be HOT) by the thermometer up at the clamp, until they are just out of the oil. This way the thermometer and sample can cool, and the hot oil can drain off. Wait for the thermometer to cool to about room temperature before you remove it entirely from the tube. Wipe off some of the oil, reload a melting point tube (never remelt melted samples), and try again. And heat at 2°C per minute this time.