By : James W Zubrick
Email: j.zubrick@hvcc.edu
ORGANIC CHEMISTRY
Table 7-4 PHYSICAL CONSTANTS OF ORGANIC COMPOUNDS
| No. | Crystalline Form and Color | Specific Gravity | Melting Point “C. | Boiling Point °C. | Solubility in 100 Parts | ||
| Water | Alcohol | Ether | |||||
| 711
712 |
![tmp30-38_thumb[4] tmp30-38_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3038_thumb4_thumb.png) |
![tmp30-39_thumb[5] tmp30-39_thumb[5]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3039_thumb5_thumb.png) |
![tmp30-40_thumb[4] tmp30-40_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3040_thumb4_thumb.png) |
![tmp30-41_thumb[4] tmp30-41_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3041_thumb4_thumb.png) |
![tmp30-42_thumb[4] tmp30-42_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3042_thumb4_thumb.png) |
![tmp30-43_thumb[4] tmp30-43_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3043_thumb4_thumb.png) |
![tmp30-44_thumb[4] tmp30-44_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3044_thumb4_thumb.png) |
![tmp30-45_thumb[3] tmp30-45_thumb[3]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3045_thumb3.png) |
![tmp30-46_thumb[3] tmp30-46_thumb[3]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3046_thumb3.png) |
||||||
Benzophenone oxide 6451
Table 7-4 PHYSICAL CONSTANTS OF ORGANIC COMPOUNDS
| No. | Crystalline Foam and Color | Specific Gravity | Melting Point °C. | Boiling Point °C. | Solubility in 100 Parts | ||
| Water | Alcohol | Ether | |||||
| 1040 | ![tmp30-47_thumb[4] tmp30-47_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3047_thumb4_thumb.png) |
![tmp30-48_thumb[4] tmp30-48_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3048_thumb4_thumb.png) |
![tmp30-49_thumb[4] tmp30-49_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3049_thumb4_thumb.png) |
![tmp30-50_thumb[4] tmp30-50_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3050_thumb4_thumb.png) |
![tmp30-51_thumb[4] tmp30-51_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3051_thumb4_thumb.png) |
![tmp30-52_thumb[4] tmp30-52_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3052_thumb4_thumb.png) |
![tmp30-53_thumb[4] tmp30-53_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3053_thumb4_thumb.png) |
| 1041 | ![tmp30-54_thumb[4] tmp30-54_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3054_thumb4_thumb.png) |
![tmp30-55_thumb[4] tmp30-55_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3055_thumb4_thumb.png) |
![tmp30-56_thumb[4] tmp30-56_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3056_thumb4_thumb.png) |
![tmp30-57_thumb[4] tmp30-57_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3057_thumb4_thumb.png) |
![tmp30-58_thumb[4] tmp30-58_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3058_thumb4_thumb.png) |
![tmp30-60_thumb[4] tmp30-60_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3060_thumb4_thumb.png) |
|
| 1057 | ![tmp30-61_thumb[4] tmp30-61_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3061_thumb4_thumb.png) |
![tmp30-62_thumb[4] tmp30-62_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3062_thumb4_thumb.png) |
![tmp30-63_thumb[4] tmp30-63_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3063_thumb4_thumb.png) |
![tmp30-64_thumb[4] tmp30-64_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3064_thumb4_thumb.png) |
![tmp30-65_thumb[4] tmp30-65_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3065_thumb4_thumb.png) |
![tmp30-66_thumb[4] tmp30-66_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3066_thumb4_thumb.png) |
![tmp30-67_thumb[4] tmp30-67_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3067_thumb4_thumb.png) |
| 1058 | ![tmp30-68_thumb[4] tmp30-68_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3068_thumb4_thumb.png) |
![tmp30-69_thumb[4] tmp30-69_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3069_thumb4_thumb.png) |
![tmp30-70_thumb[4] tmp30-70_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3070_thumb4_thumb.png) |
![tmp30-71_thumb[4] tmp30-71_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3071_thumb4_thumb.png) |
![tmp30-72_thumb[4] tmp30-72_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3072_thumb4_thumb.png) |
||
| 1059 | ![tmp30-75_thumb[4] tmp30-75_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3075_thumb4_thumb.png) |
![tmp30-76_thumb[4] tmp30-76_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3076_thumb4_thumb.png) |
![tmp30-77_thumb[4] tmp30-77_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3077_thumb4_thumb.png) |
![tmp30-79_thumb[4] tmp30-79_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3079_thumb4_thumb.png) |
![tmp30-80_thumb[4] tmp30-80_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3080_thumb4_thumb.png) |
![tmp30-81_thumb[4] tmp30-81_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3081_thumb4_thumb.png) |
|
| 1060 | ![tmp30-82_thumb[4] tmp30-82_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3082_thumb4_thumb.png) |
![tmp30-83_thumb[4] tmp30-83_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3083_thumb4_thumb.png) |
![tmp30-84_thumb[4] tmp30-84_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3084_thumb4_thumb.png) |
![tmp30-85_thumb[4] tmp30-85_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3085_thumb4_thumb.png) |
![tmp30-86_thumb[4] tmp30-86_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3086_thumb4_thumb.png) |
![tmp30-87_thumb[4] tmp30-87_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3087_thumb4_thumb.png) |
![tmp30-88_thumb[4] tmp30-88_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3088_thumb4_thumb.png) |
![tmp30-89_thumb[3] tmp30-89_thumb[3]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3089_thumb3_thumb.png) |
![tmp30-90_thumb[3] tmp30-90_thumb[3]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3090_thumb3_thumb.png) |
![tmp30-91_thumb[4] tmp30-91_thumb[4]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3091_thumb4_thumb.png) |
![tmp30-92_thumb[3] tmp30-92_thumb[3]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3092_thumb3.png) |
![tmp30-93_thumb[3] tmp30-93_thumb[3]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3093_thumb3.png) |
![tmp30-94_thumb[3] tmp30-94_thumb[3]](http://what-when-how.com/wp-content/uploads/2011/06/tmp3094_thumb3_thumb.png) |
||
| Butyl carbonate 1892-4 | Butyl citrate 6119 | Butyl cyanide (/so) 6413 |
| Butyl chlorocarbonate 1077-8 | Butyl cyanide (n) 6405 | Butyl cyanide (tert) 6246 |
2. Crystalline form . . . , mn. pr. monoclinic prisms. Here, mn is a variant of the mcl abbreviation used in the CRC. Don’t let these small differences throw you. A secret is that all handbooks have a listing of abbreviations at the front of the tables. Shhhh! Don’t tell anyone. It’s a secret.
I like the Lange’s format, redolent of the 43rd edition of the CRC. The one with the useful information. The organization based on common names, rather than systematic names, can make finding an entry a bit more difficult. There’s a miniature gloss at the bottom of each page to help you find related compounds.
Butyl carbinol(n), at the bottom of Fig. 14, has an index number of 404. If you’re familiar with the carbinol naming scheme for alcohols, it isn’t much to translate that to 1-pentanol. The entry still comes before the B’s because Amyl alcohol(n), is another common name for 1-pentanol. On the page where 1-pentanol would show up, there’s only a gloss entry: 1-Pentanol, 404. This brings you right back to Amyl alcohol(n). Since most textbooks and labbooks are making it a very big deal these days to list none but the purest of pristine systematic nomenclature, you’d likely never expect the compounds to be listed this way, and that is a bit annoying. Even though you are missing out on a bit of the history in the field.
MERCK INDEX
(The Merck Index, Merck & Co., Inc., Rahway, New Jersey.) This handbook is mostly concerned with drugs and their physiological effects. But useful information exists concerning many chemicals. Because of the nature of the listings, I’ve had to treat the explanations a bit differently than those for the other handbooks.
Entry: 1-Bromobutane (Fig. 15)
1. Top of page. 1522 n-Butylbenzene. Just like a dictionary, each page has headings directing you to the first entry on that page. So, 1522 is not the page number but the compound number for n-Butylbenzene, the first entry on page 216. The actual page number is at the bottom left of the page.
2. n-Butyl Bromide. Listed as a substituted butane with the systematic name given as a synonym.
3. C 35.06%, . . . Elemental analysis data; the percent of each element in the compound.
4. Prepd from. … A short note on how 1-bromobutane has been prepared, and references to the original literature (journals).
5. d4B 1.2686. The tiny 25 over 4 makes this a specific gravity. Note that the temperatures are given with the d and not with the numerical value as in Lange’s and the CRC.
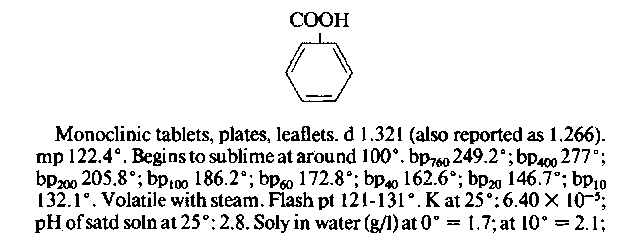
1. Line 2. dracylic acid. What a synonym! Label your benzoic acid bottles this way and no one will ever “borrow” your benzoic acid again.
2. Lines 3-7. Natural sources of benzoic acid.
3. Lines 7—9. Industrial syntheses of benzoic acid. These are usually not appropriate for your lab bench preparations.
4. Lines 9—20. References to the preparation and characteristics of benzoic acid in the original literature (journals).
5. Structure. A structural formula of benzoic acid.
6. Lines 21-40. Physical data. The usual crystalline shape, density (note two values reported.), sublimation notation, boiling point data, and so on. K at 25° is the ionization constant of the acid; the pH of the saturated solution (2.8 at 25°C) is given. The solubility data (Soly) is very complete, including water solutions at various temperatures, a bit about the phase diagram of the compound, and solubility in other solvents. Note that numerical data is given where possible.
7. Lines 41- 67. Properties of some salts of benzoic acid.
8. Line 68. Toxicity data for benzoic acid.
9. Lines 69 – 72. Some commercial uses of benzoic acid.
10. Lines 73-75. Therapeutic uses, both human and veterinary, for benzoic acid.
If all the chemical entries were as extensive as the one for benzoic acid, this would be the handbook of choice. Because benzoic acid has wide use in medi-1522 n-Butylbenzene 1526. n-Butyl Bromide. 1-Bromobutane. C„H»Br; mol wt 137.03. C 35.06%, H 6.62%, Br 58.32%. CH3(CH2)3Br. Prepd from n-butyl ale and a hydrobromic-sulfuric acid mixture: Kamm, Marvel, Org. Syn. vol. 1,5 (1921); Skau, McCullough, J. Am. Chem. Soc. 57,2440 (1935).
Fig. 15 Sample entries from the Merck Index, 10th edition.
THE ALDRICH CATALOG
(The Aldrich Catalog. Aldrich Chemical Co., Inc., Milwaukee, Wisconsin.) Not your traditional hard-bound reference handbook, but a handy book, nonetheless. The company makes many compounds, some not yet listed in the other handbooks, and often gives structures and physical constants for them. As Aldrich is in the business of selling chemicals to industry, many industrial references are given.
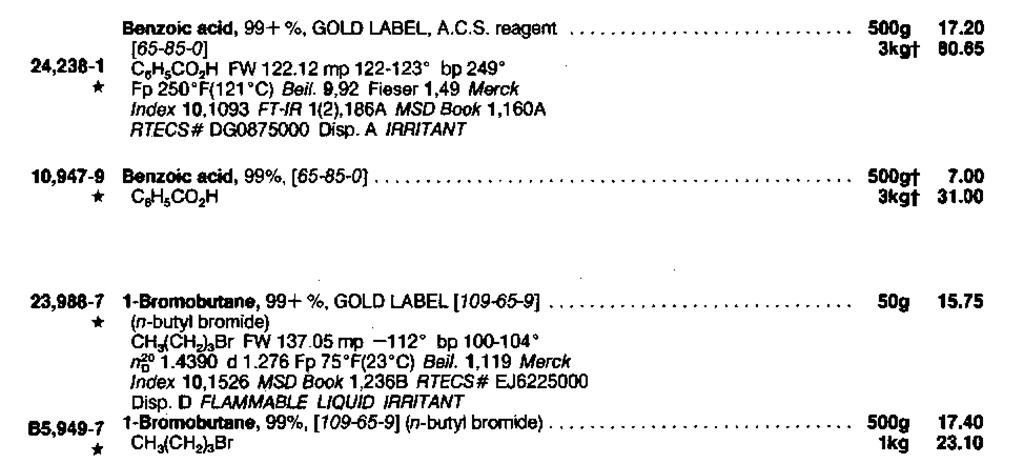
Entry: 1-Bromobutane (Fig. 16)
1. 1 -Bromobutane. Here it is listed strictly alphabetically as it is—with all the bromo-compounds—not as a butane, 1-bromo-, and only a cross reference as a butyl bromide.
2. [109-65-9].This is the Chemical Abstracts Service (CAS) Registry number. Chemical Abstracts, published by the American Chemical Society, is a listing of the abstract or summary written for any paper in the chemical literature. Every compound made gets a number. This makes for easy searching by computer, as well as by hand.
3. bp 100-104°. Without a tiny superscript this is the boiling point at 760 torr.
4. nD20 1.4390. Index of refraction. The temperature (20°) modifies the n, rather than the number as in the CRC.
5. d 1.276. The density in g/cc.
6. Fp 75°F(23°C) Flash point. Above 75°F, a mixture of 1-bromobutane and air and a spark will go up like gangbusters. Watch out!
7. Beil. 1,119. The Beilstein reference; Volume 1, page 119.
8. Merck Index 10, 1526. The Merck Index 10th ed. reference; compound #1526 (Fig. 15).
9. MSD Book 1, 236B. A reference to the page location of the entry in the Sigma-Aldrich Library of Chemical Safety Data, Edition 1.
Fig. 16 Sample entries from the Aldrich catalog, 1986-87.
10. RTECSft E J6225000. The reference number in the Registry of Toxic Effects of Chemical Substances (RTECS). 1-Bromobutane is on the inventory of the EPA according to the Toxic Substances Control Act, PL9469, October 11, 1976 (TSCA).
11. Disp D. There are methods of disposal given in the Aldrich Catalog. Go to method D and throw 1-bromobutane out according to the rules. Remember, the methods given are for the disposal of large amounts of a single substance, as might be found in an industrial application. The rules for the disposal of the waste generated in your undergraduate laboratory may differ considerably.
12. FLAMMABLE LIQUID IRRITANT. Yep, it sure is.
Note the differences in prices for the 99+% GOLD LABEL and the merely 99% 1-bromobutane. Before you buy, check on the use of the chemical. Normally, you can buy the least expensive grade of the chemical, and distill or recrystallize it yourself before you use it, if necessary.
1. Fieser 1, 49. A reference to Fieser & Fieser’s Reagents for Organic Synthesis, Volume 1, page 49. This multivolume series gives syntheses and reactions of many organic compounds, along with references to the original literature.
2. t. Benzoic acid cannot be shipped by parcel post.
3. Beil. 9, 92. A reference to Beilstein, Volume 9, page 92.
4. FT-IR 1(2) 186A. The Fourier-Transform Infra-Red spectrum of benzoic acid is in Edition 1, Volume 2, page 186A of The Aldrich Library of FT-IR Spectra.
NOT CLEAR —CLEAR?
One antonym for clear is cloudy. Another antonym for clear is colored. When you say you “obtained a clear liquid,” do you mean that it is not cloudy, or that it is colorless?
Cloudiness usually means you’ve gotten water in your organic liquid. Colorless should be self-explanatory. You should always pair the turbidity and color designations:
“a clear, colorless liquid.” “a clear, blue liquid.” “a cloudy, colorless liquid.” “a cloudy, blue liquid.”
I use clear to mean not cloudy, and water-white to mean not colored. Water-white is a designation found in the older chemical literature; colorless is more modern.
Is that clear?