Ionic Basis of the Action Potential
As stated earlier, the resting membrane potential of a neuron is usually -65 mV. At rest, Na+ influx into the neuron through open nongated Na+ channels is balanced by the efflux of K+ through open nongated K+ channels. Thus, the membrane potential remains constant closer (but not equal) to the K+ equilibrium. When a neuron receives an excitatory input, the neuronal membrane is depolarized, resulting in an opening of some voltage-gated Na+ channels and influx of Na+. It should be noted that voltage-gated Na+ channels are normally closed. The accumulation of positive charges due to influx of Na+ promotes depolarization of the neuronal membrane. When the membrane potential reaches threshold potential, the chances of generating an action potential are about 50%. However, when the membrane is depolarized beyond the threshold potential, a sufficient number of voltage-gated Na+ channels open, relative permeability of Na+ ions is greater than that of K+ ions, and action potentials are generated with certainty. Generation of an action potential is an "all-or-nothing" phenomenon. Because the concentration of Na+ channels is relatively high at the axon hillock, this is the site of generation of action potentials in a neuron.
The configuration of an action potential is shown in Figure 6-4A. During the rising phase of the action potential, there is a rapid depolarization of the membrane due to increased permeability of Na+ (Fig. 6-4B). The depolarization continues so that the membrane potential approaches the Na+ equilibrium potential. The part of the action potential in which the inside of the neuron is positive relative to the outside is called the overshoot. Toward the end of the rising phase of the action potential, voltage-gated Na+ channels are inactivated, and the influx of Na+ through these channels is stopped.
During the falling phase of the action potential, the neuron is repolarized by opening of voltage-gated K+ channels, which allows increased efflux of K+ from the neuron through these channels (Fig. 6-4B). The opening of voltage-gated K+ channels is also caused by depolarization of the neuronal membrane. Because these voltage-gated K+ channels open with a delay (about 1 msec) after the membrane depolarization, they are called delayed rectifier K+ channels. Rectifiers are channels that allow the flow of ions to move more readily in one direction than in the opposite direction. At the end of the falling phase, the membrane potential is more negative than the resting potential because of increased K+ permeability caused by the opening of the delayed rectifier K+ channels in addition to the already present resting K+ permeability through nongated channels. The permeability is closer to the equilibrium potential of K+ because there is little Na+ permeability during this period. This portion of the action potential is called after-hyperpolarization or undershoot (Fig. 6-4A). Once after-hyperpolarization has occurred, the resting membrane potential is restored gradually as the voltage-gated K+ channels close again.
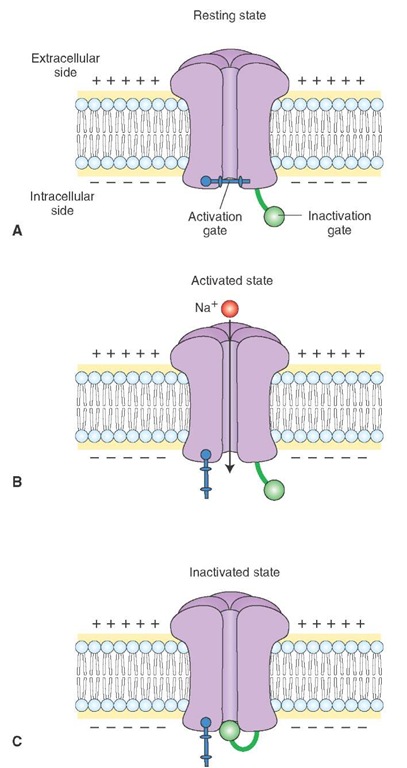
The Na+ channel has two hypothetical gates, the activation and inactivation gates. Depending on which gates are open or closed, the Na+ channel exists in the following three states: resting, activated, or inactivated.
1. Resting state: During this state, the activation gate closes the channel pore while the inactivation gate is open (Fig. 6-5A). With the channel pore closed, Na+ cannot flow into the neuron.
2. Activated state: During the rising phase of action potential, both activation and inactivation gates are open, and Na+ ions flow into the neuron (Fig. 6-5B).
3. Inactivated state: During this state, the inactivation gate closes the channel pore while the activation gate is still open (Fig. 6-5C). Even though the activation gate is open, Na+ cannot flow into the neuron. The neuron cannot be activated until the Na+ channel reverts back to resting state (i.e., the inactivation gate opens the channel, and the activation gate closes the channel). This process is known as de-inactivation. Voltage-gated Na+ channels get de-inactived when the membrane potential becomes adequately negative.
The time required for the Na+ channel to revert from inactivated to resting state (de-inactivation) determines the refractory period of the neuron. The period during which the voltage-gated Na+ channels are in inactivated state and an action potential cannot be generated is called the absolute refractory period. Immediately after the absolute refractory period (the period at the end of the falling phase), when the neuron is hyperpolarized, until the time when voltage-gated K+ channels are closed again, an action potential can be generated, but more depolarizing current is needed to shift the membrane potential to threshold level. This phase of action potential is called the relative refractory period. The entire duration of an action potential in a neuron is about 2 msec. It should be noted that, during an action potential, there is a great surge of Na+ influx into the cell via the voltage-gated Na+ channels. However, the Na+-K+ pump is working all the time, including the duration of the action potential, and transports Na+ out of the neuron via nongated channels to maintain the ionic concentration gradients across the cell membrane.
Propagation of Action Potentials
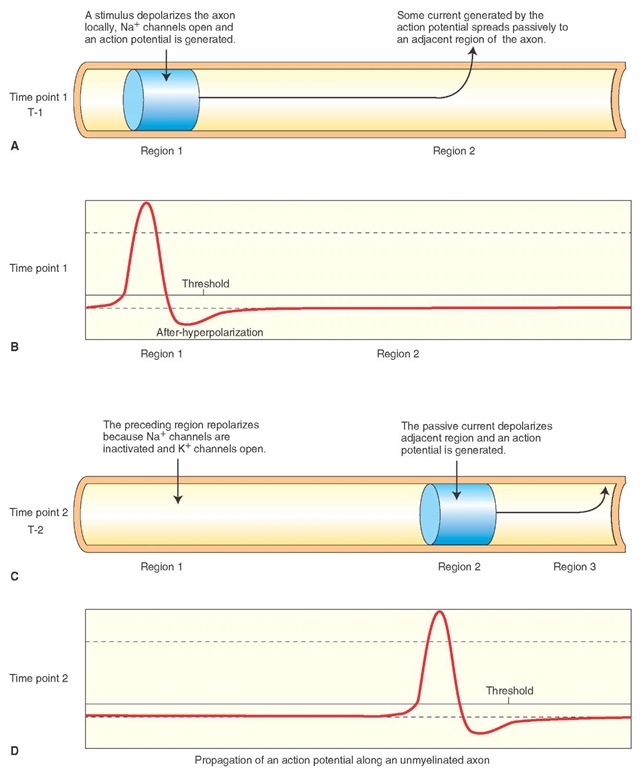
When a region (region 1 in Fig. 6-6A) of an unmyelinated axonal membrane is depolarized sufficiently by a depolarizing stimulus (e.g., a synaptic potential in a neuron) to reach a threshold potential, voltage-gated Na+ channels open, Na+ flows into the axoplasm, and an action potential is generated in that region of the axon (Fig. 6-6B).
FIGURE 6-5 Different states of voltage-gated Na+ (sodium) channel. (A) Resting state. (B) Activated state. (C) Inactivated state. Note: the "bar" is the activation gate and the "circle" represents the inactivation gate. (See text for descriptions.)
Some of the current generated by the action potential spreads by elec-trotonic conduction (passive spread) to an adjacent region (region 2 in Fig. 6-6A) of the axon. The passive spread of current occurs by movement of electrons, and movement of Na+ ions is not required. At the adjacent region, the passive spread of current results in opening of voltage-gated Na+ channels, influx of Na+ into the axoplasm (Fig.6-6C, region 2), and generation of an action potential (Fig. 6-6D). In other words, the passive spread of voltage along the length of an axon results in an active regeneration process.
FIGURE 6-6 Propagation of action potentials in unmyelinated axons, as described in the text. (A) Time point 1: Region 1 of an unmyelinated axonal membrane is depolarized. (B) Depolarization in region 1 results in an action potential at time point 1. (C) Time point 2: Passive spread of current by the action potential generated at time point 1 causes depolarization in region 2. (D) Depolarization in region 2 results in an action potential at time point 2. Na+ = sodium; K+ = potassium.
The propagation of an action potential along the axon depends on the cable properties of the axon. The larger the diameter of the axon, the lower the resistance there is to the flow of current along its length. Therefore, the conduction velocity (propagation of action potentials) along the length of the axon can be increased by increasing its diameter. For example, the axons of stellate ganglion neurons in the squid are about 1 mm in diameter (100-1,000 times larger than the axons of mammalian neurons). The conduction of action potentials in these squid giant axons is faster than that in mammalian axons. The squid needs these fast conducting axons for faster contraction of the mantle muscles that produce a jet propulsion effect needed for quick escape from predators.
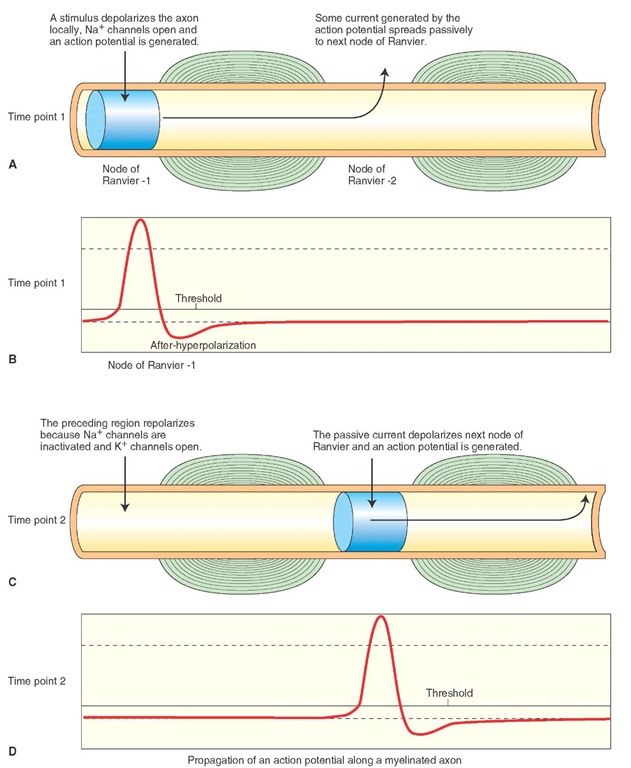
In vertebrates, the conduction velocity is increased by myelination of axon. A myelin sheath consists of about 1-mm lengths of as many as 300 concentric layers of membrane around a single axon. In the peripheral nervous system, myelin is formed by Schwann cells. In the central nervous system (CNS), oligodendrocytes form the myelin.Nodes of Ranvier (bare segments of the axonal membrane with a very high density of voltage-gated Na+ channels) are present in between the segments of the myelin sheath (Fig. 6-7, A and C). The myelinated segments of an axon are not excitable and have a high resistance to the leakage of current across them. On the other hand, passive spread of current can generate an intense current at the nodes of Ranvier due to the presence of a high density of voltage-gated Na+ channels.
FIGURE 6-7 Propagation of action potentials in myelinated axons. (A) Time point 1: A stimulus depolarizes node of Ranvier 1. (B) An action potential is generated at node 1 at time point 1. (C) Time point 2: Passive spread of current due to action potential generated at time point 1 depolarizes node 2. (D) An action potential is generated at node 2 at time point 2. Na+ = sodium; K+ = potassium.
When a depolarizing stimulus (e.g., a synaptic potential in a neuron) arrives at a node of Ranvier (node 1 in Fig. 6-7A), Na+ channels open, there is an influx of Na+ ions, and an action potential is generated at that node (Fig. 6-7B). Some current generated by the action potential spreads passively to the next node of Ranvier (node 2 in Fig. 6-7A), and depolarization of the membrane at this node results in the generation of an action potential (Fig. 6-7D). By this time, Na+ channels at the preceding node (node 1 in Fig. 6-7C) are inactivated, K+ channels open, and repolari-zation occurs. Thus, the action potential propagates along a myelinated axon by saltatory conduction (i.e., the jumping of an action potential from one node to another). Myelina-tion of an axon has two advantages: (1) conduction is very rapid along an axon, and (2) there is conservation of metabolic energy because excitation is restricted to the nodal regions that are relatively small (0.5 |im).
Clinical Considerations
Many clinical syndromes have been attributed to malfunction of different ion channels and disturbances in the generation and conduction of action potentials. Some of these syndromes are briefly described here.
Lambert-Eaton (Eaton-Lambert) Syndrome
Lambert-Eaton syndrome is usually associated with small-cell carcinoma of the lung, which is derived from primitive neuroendocrine precursor cells expressing voltage-gated Ca2+ channels. An antibody is produced in the body against these Ca2+ channels, and its presence results in a loss of voltage-gated Ca2+ channels in the presynaptic terminals at the neuromuscular junction and other synapses. Therefore, less Ca2+ enters the presynaptic terminal during depolarization and, consequently, there is a reduction in the release of the transmitter (acetylcholine [Ach]) at the neuromuscular junction that results in muscle weakness. Loss of voltage-gated Ca2+ channels at the preganglionic nerve terminals of the sympathetic and parasympathetic autonomic nervous system results in a number of symptoms characteristic of autonomic dysfunction. These symptoms include dry mouth, constipation, reduced sweating, orthostatic hypotension (dizziness while standing or walking), and impotence.
This syndrome can also occur in patients without lung cancer. In such instances a different (still unidentified) mechanism may be responsible for the loss of voltage-gated Ca2+ channels on the presynaptic terminals. Because the main symptom is muscle weakness due to insufficient Ach release, standard treatment consists of administration of drugs (e.g., guanidine and calcium gluconate) that elicit or facilitate Ach release from the presynaptic nerve terminals at the neuromuscular junction. As in myasthenia gravis, the muscle strength fluctuates with activity in Lambert-Eaton syndrome. However, in contrast to myasthenia gravis, muscle strength in these patients improves with activity because more Ach is released in response to increased activity.