Generalized Anxiety Disorder
The DSM-IV defines generalized anxiety disorder (GAD) as a condition of worry or anxiety concerning several events for the larger part of a 6-month period. There is difficulty in controlling the anxiety, and it is accompanied by difficulties in sleeping, irritability, restlessness, and muscle tension. Because the anxiety is difficult to control, it can produce impairment in everyday living. Again, this form of anxiety differs from "normal" anxiety because generalized anxiety disorder is pervasive and excessive.
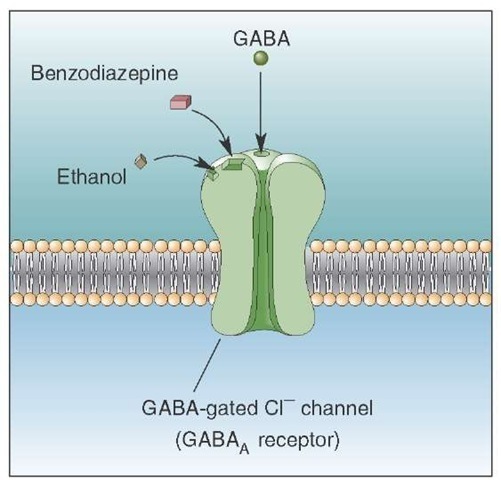
Although different classes of drugs have been used for the treatment of GAD, the drugs of choice are the ben-zodiazepines. Examples of these drugs include diazepam (Valium) and chlordiazepoxide (Librium). These drugs are effective because they interact with GABAA receptors, resulting in changes in membrane potential through the opening of Cl- (chloride) channels (Fig. 28-4). The net effect is to hyperpolarize and, ultimately, inhibit the postsynaptic neurons. Benzodiazepines increase the affinity of the GABAa receptor for GABA, causing an increase in the frequency of opening of the Cl- channel, thus facilitating GABAergic transmission. Other support for the role of the GABAergic system regulating anxiety comes from studies using inverse agonists (such as those related to P-carbolines), which bind to the benzodi-azepine site on the GABA receptor and reduce GABAer-gic transmission. In so doing, these drugs cause anxiety.
In considering other neurotransmitter systems that may relate to anxiety reactions, basic and clinical research has provided evidence that serotonin likely has a regulatory role as well. For example, buspirone (Buspar), a partial 5-HT1A agonist that has no direct effect on the GABAA-benzodi-azepine receptor and only a weak influence on dopamine receptors, has been effective in reducing, in particular, the cognitive symptoms of anxiety, as opposed to the somatic symptoms. In addition, activation of 5-HT2 receptors by a selective agonist has been shown to have anxiogenic properties, suggesting a complex relationship of the serotonergic system in its regulation of anxiety reactions.
FIGURE 28-4 Benzodiazepines, ethanol, and gamma aminobutyric acid (GABA) receptors. Benzodiazepines and ethanol act on the GABAa subunit of the GABA receptor. When GABA binds to GABAa receptors, the chloride channels open, and the influx of Cl- hyperpolarizes the cell. A benzodiazepine, such as diazepam, increases the affinity for the receptor for GABA and, consequently, increases Cl- conductance and the hyperpolarizing current. For this reason, this drug has been used successfully for the treatment of anxiety disorders.
Several neuropeptides have recently been implicated in anxiety reactions. These include CCK (see earlier discussion on panic disorder), substance P, and opiates. It appears that both CCK and substance P have anxiogenic properties, whereas opiates have anxiolytic properties. The mechanisms by which their actions modulate anxiety have not yet been elucidated. In conclusion, it would seem that anxiety reactions are not regulated by a single transmitter system but, instead, require involvement of a variety of neurotransmitters.
Substance Abuse and Brain Function
Substance abuse can be defined as the abnormal use of a substance that leads to impairment of physical, social, or occupational aspects of one’s life. Closely related to the concept of substance abuse are the concepts of dependence and tolerance. Dependence involves not only substance abuse, but also withdrawal symptoms that occur after the individual stops or reduces the intake of the substance. Tolerance is experienced when a specific dose taken previously to generate a certain kind of psychological state is no longer effective when taken subsequently. The individual thus has to increase the dose of the substance to maintain the same psychological effect of the drug.
A wide range of substances of abuse have been identified. Substances that activate central nervous system (CNS) functions, which may result in heightened alertness, agitation, mild improvement of mood, increased heart rate, loss of appetite, and aggression, are called stimulants. These include caffeine, amphetamines, cocaine, and nicotine. In contrast, agents that have a generally depressive effect on CNS functions, quite possibly by activating GABAergic inhibitory systems, are referred to as sedatives. Examples of this class of substance include alcohol, barbiturates, and benzodi-azepines. They might produce a mild elevation of mood, sedation, sleep, behavioral disinhibition, and a decrease in anxiety. Other categories of substances of abuse include: (1) narcotics or opioid drugs (e.g., heroin and morphine), and (2) hallucinogens, such as LSD, phen-cyclidine ("angel dust"), bromocriptines (when used for treating Parkinson’s disease), and cannabis (marijuana).
Neural Mechanisms
To understand how drugs of abuse generate their addictive properties, two levels of analysis have been conducted. One approach has been to attempt to identify the molecular sites for the rewarding effects of different classes of drugs. A second approach has been to identify the neural circuits mediating the rewarding effects of these drugs.
Molecular Sites
Considerable research has been carried out in attempting to identify the nature of the binding sites for opiate receptors.In brief, the -receptor is considered to be the primary site for morphine reward,tolerance, and physical dependence. For example, in knock-out transgenic mice lacking ^-receptors, the rewarding actions of morphine are absent. It is presumed that the ^.-receptor activities are mediated through different classes of G proteins, which may have different functions.
Another drug of abuse that has received considerable attention is cocaine. Available evidence points to the link between cocaine and monoaminergic transporters and, in particular, dopamine. Lesions of dopaminergic neurons that express dopamine transporters also result in the loss of the rewarding properties of cocaine. Because the effectiveness of the rewarding properties of cocaine (and also that of morphine) are associated with the rate at which they are administered, it is believed that dopamine transporter functions may be mediated through phosphorylation at specific brain sites. Although not clearly understood in terms of its neural circuitry, there is evidence that suggests that nore-pinephrine, released from neurons in the locus ceruleus, acts to facilitate the actions of neurons in the nucleus accumbens, thus promoting the reward mechanism.
The receptor mechanisms for other substances of abuse are less well understood. Although different classes of can-nabinoid receptors are found in the periphery and within the CNS, the precise regions and sites in the brain where marijuana interacts with cannabinoid receptors have yet to be elucidated. Likewise, concerning the actions of nicotine, it is believed that nicotine acts through presynaptic nico-tinic acetylcholine receptors but, again, the precise sites in the brain where such interactions take place have not been clearly identified. With respect to barbiturates and benzodi-azepines, it would appear that these classes of substances act through GABAA receptors, but the specific sites of interaction may relate more to the nature of the subunits of GABA rather than to the GABA receptor, per se. Concerning phencyclidine, it is known to bind with N-methyl-D-aspar-tate receptors of glutamate-gated ion channels, whereas LSD binds to serotonergic receptors. In contrast, alcohol’s actions may be mediated through more diverse groups of receptors. A large number of studies have provided evidence that the side effects of alcohol (ethanol) are mediated through GABA (Fig. 28-4) and glutamate receptors. However, other researchers have also suggested that monoamin-ergic and neuropeptide receptors may be implicated as well. Complicating this picture is the fact that, once alcohol passes through the blood-brain barrier, it is presumably distributed in a diffuse manner through much of the brain, acting through a variety of receptors. For this reason, it is extremely difficult to identify specific anatomical sites in the brain associated with the rewarding properties of alcohol.
Brain Circuits Mediating the Rewarding Effects of Drugs
Our knowledge of the brain sites associated with the rewarding properties (the positive, feel-good feedback that encourages use of these drugs) of different substances of abuse was facilitated by earlier studies that identified the sites that produced electrical self-stimulation in rodents. One of the most effective sites for generating electrical self-stimulation is the ventral tegmental area. Integral to the self-stimulation procedure is that the animal learns to press a bar for a reward. In this instance, the reward is a low-current stimulus applied to a specific site in the brain that is presumed to have pleasant, positive, reinforcing properties. Specifically, the neurons in the regions in question are, to a large extent, dopaminergic and project through the medial forebrain bundle to wide areas of the forebrain, including the nucleus accumbens, limbic structures, and the cerebral cortex. The following evidence implicates the ventral tegmental area as part of the brain circuit associated with rewarding drugs: (1) lesions of the medial forebrain bundle reduce the rewarding effects of drugs of abuse, such as cocaine and amphetamines; (2) these drugs potentiate the rate of electrical self-stimulation; and (3) intravenous injection of drugs of abuse, including morphine, cocaine, and amphetamines, enhances the release of dopamine at the terminals of ventral tegmental fibers, in particular, within the nucleus accumbens.
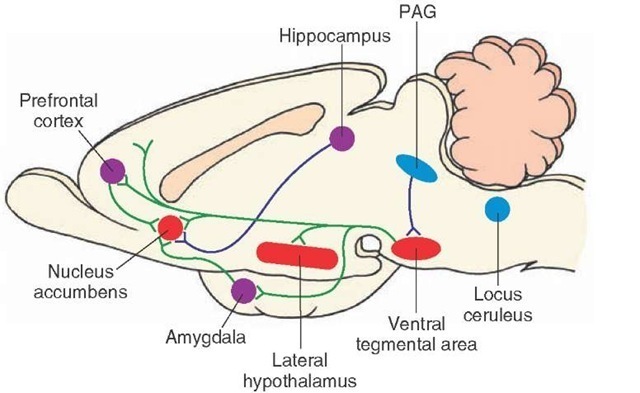
Concerning the circuitry governing the rewarding aspect of morphine, one possibility is that fibers arising from the midbrain periaqueductal gray (PAG) project to the ventral tegemental area and inhibit GABA neurons that normally inhibit dopaminergic neurons that project to wide areas of the forebrain, including the nucleus accumbens. Thus, excitation of the PAG will induce the discharge of dopamine neurons in the ventral tegmental area because of its inhibitory actions upon GABA neurons in this region. The release of dopamine into the nucleus accumbens is believed to provide the mechanism underlying the process of reward observed following the intake of selective drugs of abuse. In addition, glutamate neurons arising from regions, such as limbic structures, also project to the nucleus accumbens. The excitatory actions of these limbic neurons may serve to further drive the neurons in the nucleus accumbens to potentiate the rewarding mechanism of drugs of abuse (Fig. 28-5).
These findings suggest that different drugs of abuse may act through the same neural circuitry, although the precise mechanisms may differ. For example, certain drugs, such as cocaine, may raise the level of dopamine released at dopaminergic terminals in the nucleus accumbens by blocking the dopamine transporter, effectively extending the time for dopamine to remain in the synaptic cleft. In contrast, morphine may act on the same ventral tegmental circuit but by a different mechanism, namely, by inhibiting GABAergic neurons that normally suppress ventral teg-mental dopaminergic neurons (Fig. 28-5). Why do these drugs of abuse appear to act on the same circuit? One possible answer is that, within the brains of different organisms, there exist selective brain circuits that become activated during certain hedonistic activities such as feeding, drinking, and sexual behavior. In so doing, the activation of the brain circuits provides positive and rewarding properties in association with these motivated activities, which helps to ensure the survival of the species. Thus, drugs of abuse may be able to generate their positively rewarding properties by engaging these very same circuits.
FIGURE 28-5 A key brain-reward circuit. Intracranial self-stimulation follows stimulation along the medial forebrain bundle, which links the nucleus accumbens with the lateral hypothalamus, ventral tegmental area, amygdala, prefrontal cortex, periaqueductal gray (PAG), and brainstem monoaminer-gic cell groups. The sites where drug action is believed to occur include mainly the nucleus accumbens. Several mechanisms may be present in this scheme. One such mechanism involves an enkephalinergic projection from the PAG to the ventral tegmental area, which inhibits gamma aminobutyric acid (GABA)-ergic neurons in the ventral tegmental area (not shown in this figure) that normally inhibit dopamine neurons in the ventral tegmentum. Excitation of the PAG would, therefore, inhibit the GABAergic inhibiting mechanism and, thus, result in the release of dopamine into its target regions in the forebrain, including the nucleus accumbens. A second possibility involves inputs from limbic structures, such as the amygdala, hippocampal formation, and prefrontal cortex, that possibly use a glutamatergic mechanism. This kind of mechanism could then further excite neurons in the nucleus accumbens subserving the rewarding properties of drugs of abuse. A third element involves the possible role of the locus ceruleus, whose noradrenergic neurons project to much of the forebrain, including the nucleus accumbens. The actions of these noradrenergic neurons may serve to further potentiate the actions of neurons in the nucleus accumbens with respect to the reward mechanism.
Clinical Case
History
Harry is a 57-year-old neurologist who has worked as a faculty member at a major southeastern United States medical school for the past 20 years. He currently holds the rank of professor, which was earned by a highly productive effort in research as evidenced by continuous federal funding for his projects, by acknowledgment of his teaching skills, for which he received recognition from both students and faculty alike,and by maintaining an excellent reputation as a clinician. Harry recently accepted a position in the department as an interim chairman while a search for a permanent chairman was undertaken. Over the past few months in this role, Harry has lost significant amounts of weight and has had difficulty sleeping. In addition, he showed little interest in others, and has kept to himself. His productivity in research and clinical performance dwindled over this period as well. In recent weeks in the laboratory, he has become highly irritable, often losing his temper at the staff, and was reported to have thrown a shoe at his graduate student when the student made a mistake in the laboratory.
Examination
A friend noted Harry’s erratic behavior and recommended that he see a psychiatrist. On examination, the psychiatrist also noted that Harry displayed a flat affect and a mood of depression.The psychiatrist immediately sent him to a neurologist, who ordered a positron emission tomography (PET) scan of his head.The PET scan showed reduced blood flow, which was particularly noticeable in the prefrontal region.
Explanation
In this case, Harry’s diagnosis was depression.The PET scan record that indicated a reduced blood flow to the prefrontal region of cortex is characteristic of patients suffering from depression. For many years, catecholamines had been thought to be important in the expression of depression. Although support for this view is valid, more recent evidence accumulated in both basic and clinical research has implicated the neurotransmitter, serotonin, as playing a major role in the regulation of depressive disorders. Moreover, treatment for this disorder with serotonergic drugs has been reasonably successful.The theory, in brief, suggests that lower levels of serotonin are associated with the onset and presence of depression (and up-regulation of 5-HT receptors), whereas higher levels of serotonin tend to be linked with remission of this disorder. In addition, treatment with antidepressant drugs has been shown to result in down-regulation of 5-HT2 receptors.
Accordingly, Harry was administered a serotonin reuptake inhibitor for a period of 1 month before he began to show signs of improvement. It has been suggested that the time course of improvement after treatment can be accounted for by the time required by G proteins to affect signal transduction for modulating second-messenger systems within the cell. The presumed regions of the brain where such effects may be most significant include limbic structures and, perhaps most importantly, the prefrontal cortex. The effects of drug treatment are presumed to restore levels of serotonin and serotonin receptors to values typical of normal individuals.
After a period of 2 months, Harry resigned his position of interim chairman and again returned to the laboratory and clinic. Because of the change in his work conditions, coupled with the aid of the serotonin reuptake inhibitor and valproic acid (both of which function as mood stabilizers), his mood and temperament improved and became more stable. Likewise, he began to perform at a level similar to what he had shown before his illness. It was recommended that Harry remain on the same drug treatment regimen for the immediate future, stay away ffom chairmanships, and, at a later date, consider a gradual lowering of the drug dose overtime.
SUMMARY TABLE
Psychiatric and Behavioral Disorders: Brain Regions, Neurotransmitters, and Drug Treatment
DA = dopamine; 5-HT = serotonin; NE = norepinephrine; GABA = gamma aminobutyric acid.