Embryo Patterning and Morphogenesis
After injecting anti-miDNA-2a and anti-miDNA-13a, D. melanogaster embryos exhibited defects in the head and posterior abdominal segments, including cuticle holes and denticle belt malformations. In view of the similarity of the induced phenotypes, Boutla and colleagues (2003) concluded that these related miRNAs, miR-2a and miR-13a, act on the same target genes, together with the also related miR-2b and miR-13b, which form a functional subgroup called miRs-2/13.
Leaman and colleagues (2005) injected antisense 2′O-methyl oligoribonucleotides targeting specific miR-NAs into early embryos of D. melanogaster in order to screen the function of these miRNAs. Results showed that embryos depleted for miR-31 and miR-9 completed development, but were affected by severe segmentation defects (Figure 9). Those injected with miR-9 antisense rarely formed any trace of cuticle, and did not show internal differentiation. Embryos depleted for miR-6 were generally smaller in size than controls and had fewer and abnormally large segments, thus suggesting that apoptotic processes had been enhanced.
Sensory Organs and Functions
In D. melanogaster, miR-7 has been localized in early pho-toreceptors during embryonic eye development. At this developmental stage, miR-7 stimulates photoreceptor differentiation through a reciprocal regulation with yan, a gene encoding a transcription factor involved in the differentiation of retinal progenitor cells (Li and Carthew, 2005).
Another miRNA involved in sensory organ development is miR-9. Through both loss-of-function and gain-of-function analyses in vivo, Li and colleagues (2006) have reported that miR-9a is responsible for generating precise numbers of sensory organs in D. melanogaster embryos and adults. To accomplish this regulatory function, miR-9 a represses the translation of Senseless mRNA through its 3′ UTR region, thus ensuring a precise differential expression of this gene in sensory organ precursors and in the adjacent epithelial cells (Li et al., 2006).
Neurons that sense CO2 also provide an interesting case for study. They may have different locations depending on the species. In D. melanogaster they are located in the antenna, whereas in mosquitoes they are found in the maxillary palps. Cayirlioglu and colleagues (2008) observed that loss of miR-279 in D. melanogaster determines that CO2 neurons change their location from the antennae to the maxillary palps. The authors suggest that miR-279 downregulates Nerfin-1, a specific target for this miRNA, thus preventing the development of CO2 neurons in the maxillary palps (Cayirlioglu et al., 2008).
An example of miRNA that ensures developmental robustness during apoptotic tissue pruning is miR-263a/b (Hilgers et al., 2010), which protects sense organs during apoptosis by directly acting upon, and limiting the expression of, the pro-apoptotic gene hid. This property of some miRNAs to buffer fluctuating levels of gene activity makes them well suited to serve a protective function during development (Hilgers et al., 2010).
Figure 9 Effects of depletion of miR-31 in Drosophila melanogaster embryos. (A) and (E) show Darkfield images of cuticle preparations; (B), (C), (F), and (G) are confocal images of Eve (red) and Ftz (green) stainings; and (D) and (H) hairy RNA in situ hybridization of blastoderm (2.5-h) embryos. (A)-(D) correspond to controls, and (E)-(H) to miR-31 antisense-injected embryos. The latter show cuticle defects ranging from partial fusion to complete loss of segments (E). In controls (B) and (C), Eve and Ftz are expressed in seven largely non-overlapping stripes, while miR-31 antisense-injected embryos (F) and (G) show fewer and weaker stripes that often bleed into each other. The hairy transcript pattern also shows fewer stripes (H), indicating that pattern formation is affected upstream of the primary pair rule genes.
Muscle Differentiation
In D. melanogaster, miR-1, which is one of the best conserved miRNAs in animals, is specifically expressed in the mesoderm during early embryogenesis, and in myo-genic precursors and muscle cells in late embryos (Sokol and Ambros, 2005). Depletion of miR-1 using genetic approaches, or by treatment with 2′O-methyl antisense oligonucleotides, resulted in lethality, which implies that miR-1 has essential functions in mesodermally derived tissues (Nguyen and Frasch, 2006).
By analyzing D. melanogaster mutants devoid of miR-1, Kwon et al. (2005) assessed the essential role of miR-1 for muscle differentiation. They showed that miR-1 regulates the determination of specific cardiac and somatic muscle lineages from pluripotent progenitor cells in early embryogenesis. The Delta protein, a ligand for the Notch signaling pathway, was identified as an miR-1 target in cardiac progenitor cells (Kwon et al., 2005).
Another well-conserved miRNA is miR-133, which is expressed in muscle cells together with miR-1. In D. melanogaster embryos, miR-133 plays a key role in controlling alternative splicing during muscle formation, and defining the properties of differentiated muscle cells, through repressing the expression of the splicing factor nPTB during myoblast differentiation into myotubes (Boutz et al., 2007). The results of Boutz and colleagues not only indicate miR-133 directly downregulates a key factor during muscle development, but also establish a role for microRNAs in the control of a developmentally dynamic splicing program.
Growth
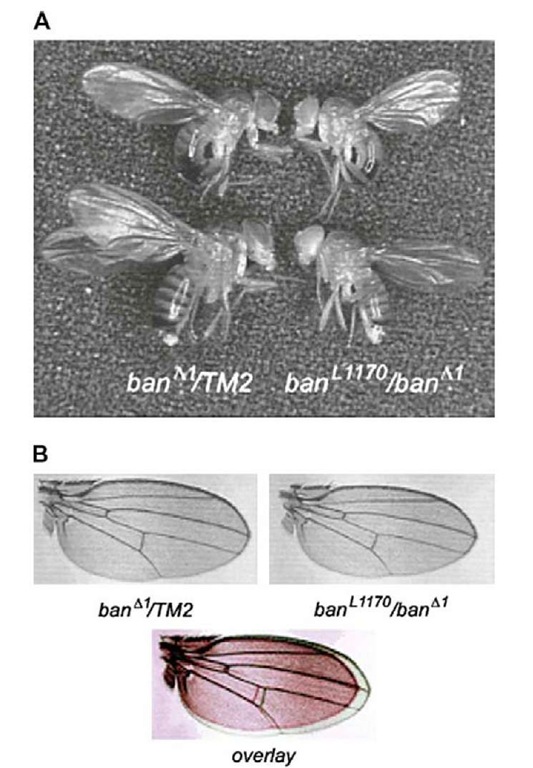
In D. melanogaster, loss-of-function mutations of the bantam locus are lethal at the early pupal stage, whereas hypomorphic combinations of bantam mutant alleles give rise to adult flies that are smaller than controls (Figure 10) and that have deficiencies in fertility (Hipfner et al., 2002). Conversely, overexpression of bantam induces tissue overgrowth due to an increase in cell number. Bantam expression appears to be regulated by the gene Yorkie, thus controlling organ growth during development (Edgar, 2006; Thompson and Cohen, 2006).
Related to growth, and also in D. melanogaster, miR-278 has been implicated in insulin receptor (InR) signaling, thus contributing to regulation of the energy balance mainly by controling insulin responsiveness. Overex-pression of miR-278 promotes tissue growth in the eye and wing imaginal disks, whereas its deficiency leads to a reduction of fat body mass, which is reminiscent of the effect of impaired InR signaling in adipose tissue; the action of miR-278 could be produced through the regulation of expanded gene transcripts (Teleman et al., 2006).
More recently, Hyun and colleagues (2009) have reported that miR-8 and its target, U-shaped (USH), regulate body size in D. melanogaster. miR-8 null flies are smaller in size and defective in insulin signaling in the fat body. USH inhibits PI3K activity, thus suppressing cell growth. Fat-body-specific expression and clonal analyses showed that miR-8 activates PI3K, thereby promoting fat-cell growth cell-autonomously, and enhancing organ-ismal growth non-cell-autonomously (Hyun et al., 2009).
Metamorphosis: Ecdysteroids and Juvenile Hormone
In insects, molting and metamorphosis are controlled by juvenile hormones and ecdysteroids, usually 20-hydroxy-ecdysone. Simultaneous expression of miR-125 and let-7 during D. melanogaster post-embryonic development is synchronized with the high titer of ecdysteroid pulses that initiate metamorphosis (Bashirullah et al., 2003; Sempere et al., 2003), which suggests that ecdysteroids might regulate the expression of these two miRNAs.
Bashirullah and colleagues (2003), however, showed that miR-125 and let-7 expression is neither dependent on the Ecdysone receptor (EcR) nor inducible by 20-hydroxyecdysone in larval organs incubated in vitro. The same authors reported that the expression of both miRNAs can be induced by 20-hydroxyecdysone in Dro-sophila Kc cells, although the induction is considerably delayed with respect to what is observed in vivo (Bashirul-lah et al., 2003). ‘The conclusion of these experiments is that the action of 20-hydroxyecdysone in Kc cells might be indirect, and that miR-125 and let-7 should be directly induced by an unknown temporal signal distinct from the well-known ecdysteroid-EcR cascade.
In a parallel paper, Sempere and colleagues (2003) followed a different approach to study the influence of ecdysteroids on the expression of miR-125, let-7, and miR-100, which are upregulated after the ecdysteroid pulse, as well as of miR-34, which is downregulated. They used the temperature-sensitive ecd1 strain that is impaired in ecdysteroid synthesis, and they showed that in ecd1 specimens blocked from pupariation by a transfer at 29°C, miR-125, let-7, and miR-100 were detected at much lower levels, whereas miR-34 was detected at much higher levels, compared with the wild type. Sempere and colleagues (2003) also studied the possible role of Broad complex, an early inducible gene in the ecdysteroid cascade, using npr6 specimens, which lack all Broad complex factors. Results showed that miR-125, let-7, and miR-100 were detected at much lower levels (and miR-34 at much higher levels) in homozygous npr6 specimens than in npr6/+ or wild type specimens. With the same experimental approach, Sempere and colleagues (2003) concluded that ecdysteroids and Broad complex activity are required for temporal upregulation of miR-125, let-7, and miR-100, and downregulation of miR-34.
Figure 10 In Drosophila melanogaster, loss-of-function mutations of the bantam locus are lethal at the early pupal stage, whereas hypomorphic combinations of bantam mutant alleles give rise to adult flies that are smaller than controls. Panel (A) shows the reduced body size of male and female flies of banU170/banA1 (defective for bantam), with respect to banA1/TM2 siblings (control). Panel (B) compares wing sizes in both combinations. In the overlay, the banL1170/banA1 wing is shown in red and the banA1/TM2 wing in green.
Additional experiments carried out by these authors with Drosophila S2 cells showed that incubation times longer than 30 h with 20-hydroxyecdysone correlated with increased levels of miR-125, let-7, and miR-100, whereas miR-34 was detected at very low levels at all times studied. Moreover, the addition of methoprene, a juvenile hormone analog, enhanced the expression of miR-34 and reduced the ecdysteroid-stimulatory effect on the expression of miR-125, let-7, and miR-100 (Sempere et al., 2003). In these experiments, Broad complex was shown to be necessary for enhancing the activity of 20-hydroxyecdysone.
Discrepancies between the two papers (Bashirullah et al., 2003; Sempere et al., 2003) look more apparent than real, given that the respective sets of results, which emerge from quite different experimental approaches, are not incompatible. Indeed, those of Sempere and colleagues (2003) do not discard an indirect action of ecdys-teroids, which is the hypothesis postulated by Bashirullah and colleagues (2003).
A miRNA clearly associated to ecdysteroid pulses in D. melanogaster is miR-14. In this fly, ecdysteroid signaling through the EcR seems to act via a positive auto-regulatory loop that increases EcR levels, thus optimizing the effect of ecdysteroid pulses. In this context, miR-14 modulates this loop by limiting the expression of EcR, whose mRNA contains three miR-14 sites in the 3′ UTR. In turn, ecdysteroid signaling, through EcR, downregu-lates miR-14. This modulatory action of miR-14 may be crucial due to the intrinsic lability of the positive autorregulatory loop that controls ecdysteroid signaling (Varghese and Cohen, 2007).
Metamorphosis: Morphogenesis
Work by Sokol and colleagues (2008) showed that the D. melanogaster let-7-Complex locus (let-7-C, comprising let-7, miR-100, and miR-125; see Figure 3) is mainly expressed in the pupal and adult neuromusculature. let-7-C knockout flies look morphologically normal, but display defects in different adult behaviors (like flight and motility) and in fertility. Importantly, their neuromus-culature clearly shows juvenile features, which suggests that an important function of let-7-C is to ensure the appropriate remodeling of the abdominal neuromuscula-ture during the larval-to-adult transition. The study also showed that this function is carried out predominantly by let-7 alone (Sokol et al., 2008).
In a related work, Caygill and Johnston (2008) obtained a D. melanogaster mutant that lacks let-7 and miR-125 activities and shows a pleiotropic phenotype that arises during metamorphosis. These authors showed that the loss of let-7 and miR-125 results in temporal delays in the terminal cell-cycle exit in the wing, and in the maturation of neuromuscular junctions of imaginal abdominal muscles. The authors focused on the latter process by identifying the abrupt (ab) gene (which encodes a nuclear protein) as a let-7 target, and by providing evidence showing that let-7 regulates the maturation rate of abdominal neuro-muscular junctions during metamorphosis by regulating ab expression (Caygill and Johnston, 2008).
Wing morphogenesis has been studied by Biryukova and colleagues (2009), who described that miR-9a regulates D. melanogaster wing development through a functional target site in the 3′ UTR of the LIM only (dLOM) mRNA. dLMO is a transcription cofactor that directly inhibits the activity of Apterous, the factor required for the proper wing dorsal identity. Deletions of the 3′ UTR that remove the miR-9a site generate gain-of-function dLMO mutants associated with high levels of dLMO mRNA and protein. These mutants lack wing margins, a phenotype that is characteristic of null miR-9a mutants. Of note, miR-9a and dLMO are co-expressed in wing disks and interact genetically for controlling wing development; thus, the absence of miR-9a results in overexpression of dLMO, while gain-of-function miR-9a mutant suppresses dLMO expression. The data suggest that miR-9a ensures a precise dosage of dLMO during D. melanogaster wing development (Biryukova et al., 2009).
Another miRNA involved in wing morphogenesis of D. melanogaster is iab-4. Sequence analysis suggested that iab-4 could regulate Ultrabithorax (Ubx), and expression pattern studies of iab-4 and Ubx showed that they are complementary in critical developmental moments. Direct evidence for an interaction between iab-4 and Ubx was obtained with luciferase assays. Finally, ectopic expression of iab-4 miRNA in haltere disks caused a homeotic transformation of halteres to wings, which occurs when Ubx expression is reduced (Ronshaugen et al., 2005).
As stated above, RNAi experiments that reduced Dicer-1 expression in the last instar nymph of B. germanica depleted miRNA levels, and the next molt, instead of giving the adult stage, gave supernumerary nymphs. These were morphologically similar to the supernumerary nymphs obtained after treating the last instar nymph with juvenile hormone (Figure 11). The RNAi experiments with Dicer-1 indicate that miRNAs are crucial for hemimetabolan metamorphosis (Gomez-Orte and Belles, 2009).
Behavior
A recent paper by Kadener and colleagues (2009a) addresses the contribution of miRNAs to the regulation of circadian rhythms. The authors first knocked down the miRNA biogenesis pathway in D. melanogaster circadian tissues, which severely affected behavioral rhythms, thus indicating that miRNAs function in circadian timekeeping. To identify miRNA-mRNA pairs that might be important for this regulation, immunoprecipitation of Ago-1, followed by microarray analysis, led to identification of a number of mRNAs presumably under miRNA control. These included three core clock mRNAs: clock; vrille; and clockworkorange. To identify miRNAs involved in circadian timekeeping, the authors inhibited miRNA biogenesis in circadian tissues and then carried out a tiling array analysis. Behavioral and molecular experiments showed that bantam has a role in the core circadian pacemaker, and S2 cell biochemical assays indicated that bantam regulates the translation of clock by targeting three sites in the clock 3′ UTR (Kadener et al., 2009a).
In a work addressed to study of the role of miR-8 in D. melanogaster, Karres and colleagues (2007) identified atrophin (also known as grunge) as a direct target of miR-8. miR-8 mutant phenotypes show high levels of apoptosis in the brain, and behavioral defects, like impaired capability for climbing, which are attributable to elevated atrophin activity. Decrease of atrophin levels in miR-8-expressing cells to below the level generated by miR-8 regulation is detrimental, which points to a sort of "tuning target" relationship between them (Karres et al., 2007).
Polyphenism, Caste Differentiation, and Sexual Differences
Legeai and colleagues (2010) suggested that miRNA might participate in the regulation of aphid polyphenism, and studied the expression of miRNA in different female morphs of A. pisum using microarray approaches. Most (95%) of the miRNA tested (n = 149) had similar expression in different morphs, but some of them, including miR*s, were differentially expressed — like let-7 and miR-100, which were upregulated in oviparae specimens, and miR2a-1, which was downregulated.
Figure 11 Inhibition of Blattella germanica metamorphosis after impairing miRNA maturation by depleting Dicer-1 expression with RNAi approaches in sixth (last) instar nymph. Dorsal and ventral view of normal sixth instar nymph (A, B), normal adult (C, D) and seventh instar supernumerary nymphoid (E, F) resulting from metamorphosis inhibition. The nymphoids resemble those obtained after treating the last instar nymph with juvenile hormone (G).
The comparison between two parthenogenetic morphs gave three miR-NAs (miR-34, miR-X47, and miR-X103) and two miR*s (miR307* and miRX52*) that showed differential expression. While miR307* was upregulated in virginoparae, the others were downregulated with respect to the sexu-parae morph.
Using total reads from Solexa deep sequencing, Wei and colleagues (2009) compared miRNA expression in the gregarious and solitary phases of the migratory locust, L. migratoria. In the gregarious phase, canonical miRNAs were expressed at levels between 1.5- and 2-fold higher than in the solitary phase; the most prominent differences were found in miR-276, miR-125, miR-1, let-7, and miR-315. Interestingly, miR-1 is a muscle-specific miRNA and miR-315 is a potent Wingless signaling activator, at least in D. melanogaster; therefore, the differences in flying power between gregarious and solitary locusts may be related by the action of these two miRNAs. However, most of the differences concern new unanno-tated small RNAs, which are much more abundant in the solitary phase, although the functions of these miRNA candidates remain unknown.
In the honey bee A. mellifera, expression profiles of miRNAs in workers and queens have been compared using quantitative RT-PCR, in adult body parts (head, abdomen, thorax) as well as in the whole body of the pupal stage (Weaver et al., 2007). Results highlighted the differential expression, between queens and workers, in the abdomen, which is probably related to the location of the ovaries and the differential fecundity of the two castes. Regarding particular miRNAs, miR-71 shows strong expression in worker pupae; comparing adult body parts, miR-71 has a higher expression in the head and thorax of adult workers, whereas expression in the abdomen is higher in the queen caste. Conversely, miR-9a is highly expressed in the thorax and abdomen of workers, while their expression levels are the highest in the thorax of workers.
In the silkworm B. mori, Liu and colleagues studied the expression of miRNA adult males and females using microarray approaches, and found that the expression of some 20 miRNAs was significantly higher in the body wall of males (S. Liu et al., 2010). This differential expression was assessed for a selection of 10 miRNAs (including bantam, miR-1, miR-13a, and miR-2a) using Northern blot. Microarray analysis also revealed that the expression of 13 miRNAs was significantly higher in ovaries, whereas only 4 were differentially expressed in testes. Differences between sexes were also found in other tissues, including Malpighian tubules, head, midgut, fat body, or silk gland. However, the authors pointed out that differences in miRNA expression might be due to individual differences in the metabolic state, because the expression of some of these miRNAs is influenced by nutritional status (Cheung et al., 2009).
Response to Biological Stress
Larvae of the moth Lymantria dispar show differentiated miRNA expression after wasp parasitization (Gundersen-Rindal and Pedroni, 2010). Microarray studies revealed that miR-1, miR-184, and miR-277 are highly upregulated in larval hemocytes, whereas miR-279 and let-7 are highly downregulated. Expression changes were assessed in hemolymph, fat body, brain, and mid-gut from infected larva, with respect to controls, using qRT-PCR. Of all the tissues analyzed from parasitized specimens, the midgut was the one that showed least miRNA activity. miR-1 was upregulated in all tissues from parasitized specimens, whereas miR-277 was the most strongly upregulated in the fat body. Expression of miR-279 was variable in the different tissues; it was remarkably upregulated in fat body but clearly downregu-lated in hemolymph, and it had a negligible expression in brain and midgut. Two human herpes virus-associated miRNAs (hcmv-miR-UL70 3p and kshv-miR-K12-3) were upregulated in hemocytes of parasitized L. dispar (Gundersen-Rindal and Pedroni, 2010). These are among many miRNAs that have been hypothesized to act as suppressors of the immediate and early genes that respond to a viral infection (Murphy et al., 2008).
The response of miRNAs to an infection has also been studied in mosquitoes. In A. gambiae, expression of miR-34, miR-1174, and miR-1175 decreases after Plasmodium infection, while that of miR-989 increases (Winter et al., 2007). Minor changes in miRNA expression have been observed in C. quinquefasciatus after West Nile virus infection (Skalsky et al., 2010), although miR-989 showed a 2.8-fold downregulation and miR-92 appeared somewhat upregulated. The expression of these two miR-NAs has been studied in different mosquito species, and results have shown that miR-989 expression is restricted to females, and predominantly to the ovary (Winter et al., 2007; Mead and Tu, 2008), although it was later detected in the midgut of Ae. aegypti (S. Li et al., 2009). miR-92 has been related to embryonic development in Ae. aegypti (S. Li et al, 2009) and B. mori (Liu et al, 2009). Results of deregulation of miR-989 and miR-92 suggested to Skalsky and colleagues (2010) that their targets participate in mediating flavivirus infection of the mosquito host.
Apoptosis
A group of D. melanogaster miRNAs including, miR-278, miR-14, bantam, and miR-2, regulate cell proliferation and apoptosis, targeting a number of pro-apoptotic genes, like hid, which is repressed by bantam and miR-2 miRNAs (Brennecke et al, 2003, 2005; Stark et al, 2005).
One of the functions of mir-14 in D. melanogaster is suppressing cell death; therefore, loss of miR-14 is associated with a reduced lifespan, stress sensitivity, and increased levels of the apoptotic effector caspase Drice (Xu et al., 2003). The same anti-apoptotic function has been found in Lepidopteran (Spodoptera frugiperda) Sf9 cells, where miR-14 is required for constitutive cell survival (Kumarswamy and Chandna, 2010). However, the results do not exclude that additional miRNAs might also contribute to regulating Lepidopteran cell survival and death.
Finally, experiments depleting miRNA functions by injection of miRNA antisense nucleic acids in early embryos, which permits systematic loss-of-function analysis in vivo, have identified the miR-2/13 family and miR-6 as controlling apoptosis during D. melanogaster embryonic development through post-transcriptional repression of the proapoptotic proteins hid, grim, reaper, and sickle (Leaman et al., 2005).
Conclusions and Perspectives
There are reasons to believe that the still-scarce data available on miRNAs are just the tip of an iceberg. Nevertheless, rapidly expanding information is making it increasingly obvious that miRNAs’ contribution to the genomic output is not a sort of genetic oddity or "transcriptional background noise," but a class of key post-transcriptional regulators of gene expression. Indeed, genomic regulation cannot be completely understood without incorporating the role of miRNAs, which constitute a regulatory layer that works in concert with the mRNA and protein network. However, the field of miRNA study is still in the development phase, and there are many aspects — the bulk of the iceberg — that require further research.
The miRNA machinery appears to be more complex than previously thought, and rapid progress is unveiling many unexpected details. An example concerning the mechanisms responsible for stabilized or reduced miRNA expression is the discovery of specific cis-acting modifications and trans-acting proteins that affect miRNA half-life, which are revealing new elements that contribute to their homeostasis (Kai and Pasquinelli, 2010), and the identification of Dicer-independent miRNA biogenesis pathways, such as those using the catalytic activity of Ago-2 (Cheloufi et al, 2010; Cifuentes et al, 2010). Therefore, recent data suggest that such mechanistic aspects will have more surprises revealed as we continue to expand our understanding of them.
Moreover, further studies are required to elucidate how miRNA genes are regulated. There are contributions studying the influence of transcription factors acting on the promoter region of miRNA genes, like that of Pek and colleagues (2009) on the aforementioned repres-sor action of Maelstrom on the miR-7 promoter region. However, more studies in this line are needed if we wish to better understand the regulatory mechanisms mediated by miRNAs. Faster progress seems predictable in the field of cataloging miRNAs. The challenge is to find the unexpected, non-conserved miRNAs, and the new generation of algorithms will have to combine not only high-throughput approaches and powerful computational methods, but also expression data, genomic location, and structural and sequence features, as approached in the studies of Brennecke and Cohen (2003), Friedlander and colleagues (2008), and Mathelier and Carbone (2010).
miRNA target prediction started more than two decades ago with the serendipitous findings that emerged from miRNA target recognition (Wightman et al, 1991, 1993; Lee et al., 1993). The key principles were then applied to computational methods for miRNA target prediction (Bartel, 2009), and these methods soon allowed the prediction of hundreds of miRNA targets. However, computational prediction of miRNA targets still relies on the few principles defined more than 20 years ago, and, arguably, this will not help to unveil novel aspects of miRNA target mechanisms. Thus, unbiased approaches to studying the interaction of miRNA and target would be valuable in order to identify new principles of miRNA-target recognition, and to improve the systems for target prediction, as in the creative approach of Orom and Lund (2007).
Finally, the phase of predicting putative targets in silico following computational methods must be followed by experimental work to validate the predictions and to identify targets in vivo. In this line, specific miRNA silencing will be one of the most useful approaches, and entomologists and other non-biomedical researchers will benefit from the miRNA antagonists that are being designed in the context of biomedical studies in search of therapeutic agents against human diseases (Gao and Huang, 2009). In this sense, D. melanogaster and other insect species will continue to be the favorite models, given the advantages they offer, especially straightforward manipulation. Validation of targets will contribute to elucidating the place and role of miRNAs in the molecular network that regulates the development and homeostasis of biological processes. The networks describing the interaction of miRNAs, mRNAs, and proteins are presumably highly organized and complex, and their their study therefore represents a formidable challenge. However, it will be a worthwhile effort, because these networks are possibly the best approximation to the living world that is available with present means.
There is a fairly widespread opinion that proteins are what really matter in the functional landscape that shapes fitness, so transcript abundance is only useful as a mere proxy for the activity of the corresponding proteins (Feder and Walser, 2005). However, the expanding universe of small silencing RNAs (Ghildiyal and Zamore, 2009) and their widespread functional roles in genomic regulation show this to be yet one more old paradigm that is about to fall.