13.5.
Cell Material and Action
A simple storage battery element is made of two dissimilar metal plates (called electrodes) that are kept from touching each other by a separator. This element is submerged in a liquid sulphuric acid electrolyte solution. An electrolyte is a material whose atoms are free to move about in the solution. The acidity of the electrolyte weakens the electron bonds of the plate materials, so that the electrons can drift, causing positive and negative ions to be formed in the plate material.
The active material on one of the plates is lead dioxide, usually called lead peroxide (Pb02), a dark brown, small-grain crystalline material. The crystalline type of structure is very
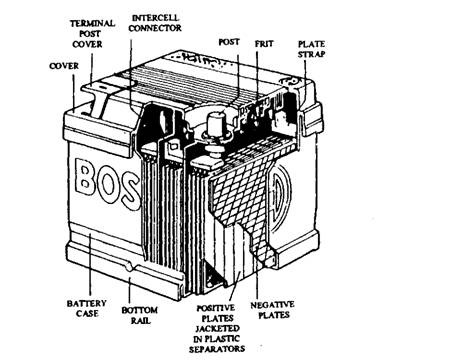
Fig. 13.53. A lead-acid battery.
porous, so the electrolyte can freely penetrate the plate. Electrons leave the lead peroxide plate, leaving positive ions behind in the plate and enter the electrolyte.
The active material on the other plate is porous or sponge lead that is also easily penetrated by electrolyte. Electrons leave the electrolyte and enter the lead, giving rise to accumulation of excess electrons on sponge lead plate that produce negative ions in the plate, shown in Fig. 13.54.
The electromotive force between the lead peroxide plate and sponge-lead plate is 2.13 V. Cell voltage is the result of the type of materials used in the plates and not the plate size, shape, or number of plates in a cell. If the plates are connected by a
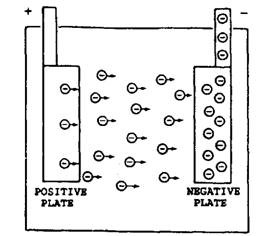
Fig. 13.54. Electron movement within a cell.
conductor outside the cell, electrons can leave the negative plate and flow through the conductor to the positive plate as shown in Fig. 13.55. This process continues as long as the chemical action
within the cell transfers electrons to the negative plate. This process is called discharging the cell.
13.5.1. Cell Electrochemical Action
A fully charged lead-acid battery contains lead peroxide (PbC”2) positive plate, spongy lead (Pb) negative plates and dilute sulphuric acid (H2SO4 + H2O) called electrolyte. The dilution of the electrolyte is indicated by its relative density (specific gravity) which is 1.28 for fully charged state. The lead is known as the active material, which has different valencies in its two forms. The lead has a vallency of + 4 in lead peroxide. This means a different number of electrons exist in the outer shell of the pure lead than in the lead peroxide.
The sulphuric acid being in an aqueous solution (i.e. mixed with water) dissociates into charged ions : H+, H+ and SOf~. From outside the polarity of the electrolyte appears to be neutral, as these charges cancel out. This dissociation of the electrolyte causes the flow of a charging or discharging current through the liquid.
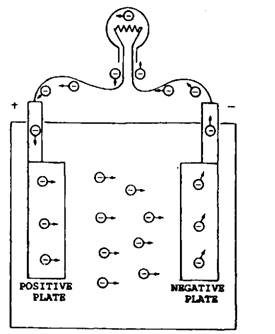
Fig. 13.55. Electron movement in a complete circuit.
The voltage of a cell is created by the ions (charged particles), which are forced into the solution from the electrode by the solution pressure. Lead gives up two positively charged atoms, which release two electrons into the liquid. Consequently after giving up two positively charged particles the electrode now has an excess of electrons and hence takes on a negative polarity with respect to the electrolyte. If a further electrode is immersed into the electrolyte, different potentials develop at the two electrodes and therefore a potential difference exists between the
two. A lead-acid battery has a nominal potential difference of 2V. The electrical pressu re now offered by the plates results in an equilibrium within the electrolyte. This is because the nt rative charges on one plate exert an attraction on the positive ions, which have entered the solu’ on. This attraction has the same magnitude as the solution pressure and hence equilibrium Ki maintained. When an external circuit is connected to the cell the solution pressure and attraction force are disrupted so that additional charged particles are passed into and through the electrolyte. This happens only if the external voltage pressure is greater than the electrical tension within the cell. This in fact is known as the charging voltage.
During discharge, excess electrons leave the sponge-lead plate through the exterior conductor, leaving positive lead ions (Pb2+) on the plate. Negative sulphate ions (SOl~) from the electrolyte are attracted by the positive lead ions. They combine to form neutral lead sulphate (PbS04) on the negative plate. During this time, the lead peroxide (Pb02) of the positive plate
combines with hydrogen (H+) from the electrolyte to release electrons as a positive lead ion
(Pb2+), and water (H2O) is formed. The positive lead ions from this reaction combine with
negative sulphate ion (SO|~) of the electrolyte to form neutral lead sulphate PbS04 on the positive plate. Figure 13.56 shows schematically above ion movements. In an ideal situation, if a cell is completely discharged, both the plates become lead sulphate and the electrolyte becomes water. The specific gravity of the electrolyte indicates the amount of electrical activity remaining in the cell. A fully charged electrolyte has a specific gravity of 1.28.
In lead acid battery the electrochemical process is reversible because lead sulphate is very slightly soluble and remains on the plate. If a cell is connected to a voltage higher than the cell voltage, electrons flow backward through the cell causing a reverse chemical action, due to which sulphate ions leave the plates and re-enter the electrolyte. The plates again become lead and lead peroxide. The cells are slowly charged or discharged because faster reaction results in the formation of excess hydrogen and oxygen gases, which leave the cell through the vent. As the cell reaches fully charged state hydrogen and oxygen gases are formed respectively at the

Fig. 13.56. Chemical ion movement in a cell during discharge.
negative and positive plates. In practice the cells are seldom fully discharged, but are usually kept near full charge.
When a lead acid cell is undergoing charging or discharging certain chemical changes take place. This can be considered as two reactions, one at the positive plate and one at the negative plate. At the positive plate, the lead peroxide combines with the dissociated hydrogen and tends to become lead and water.
![clip_image002[8] clip_image002[8]](http://lh4.ggpht.com/_Ii1ukGkfijY/Sqn4ojFIzaI/AAAAAAAACv8/a4UdenC7tYY/clip_image0028_thumb.jpg?imgmax=800)
The other reaction that takes place in a battery is gassing after the battery fully charged. This occurs because once the plates of the battery have become pure lead and lead peroxide ; the external electrical supply decomposes the water in the electrolyte. The gassing voltage for a lead-acid battery is about 2.4 V. The decomposition of water causes hydrogen and oxygen to be given off resulting in loss of water (H2O), and an equally undesirable increase in electrolyte acid density.
![clip_image002[10] clip_image002[10]](http://lh5.ggpht.com/_Ii1ukGkfijY/Sqn4qftG1sI/AAAAAAAACwE/y1WvKlLlTvU/clip_image00210_thumb.jpg?imgmax=800)
The gassing should occur only for a short time, just to ensure that all the lead sulphate has been converted to either lead or lead peroxide. The material of the grids inside a battery contributes to the gassing. With sealed batteries this causes a problem, which has been resolved to a large extent by using lead-calcium for the grid material in place of the more traditional lead-antimony.
Table 13.4. Cell and Battery Voltage at Various Acid Densities. |
|||
| Acid density | Cell voltage | Battery voltage | % charge |
| 1.28 | 2.12 V | 12.7 V | 100 |
| 1.24 | 2.08 V | 12.5 V | 70 |
| 1.20 | 2.04 V | 12.3 V | 50 |
| 1.15 | 1.99 V | 12.0 V | 20 |
| 1.12 | 1.96 V | 11.8. V | 0 |
The voltage of a cell and hence the status of battery is largely determined by the concentration of the acid in the electrolyte. The temperature also offers a remarkable effect. These figures can be calculated from the mean electrical tension of the plates and the concentration of ions in solution. The results of these calculations at 300 K are listed in Table 13.4. For a rough estimation the cell voltage is about 0.84 plus the value of the relative density. The terminal voltage of a lead-acid cell must not fall below 1.8 V, because apart from the electrolyte tending to become very close to pure water, the lead sulphate crystals grow markedly, so that it becomes difficult to recharge the battery.
