Introduction
Entamoeba histolytica is a protozoan parasite that causes amebic dysentery and liver abscess. The disease is common in tropical regions of the world where hygiene and sanitation is often approximate. The epidemiology of E. histolytica has been studied around the world. However, there is a dearth of comprehensive literature on the epidemiology of this pathogen as well as its pathogenicity in the tropical and underdeveloped regions of the world where the disease is actually more common. Epidemiological figures in many endemic tropical countries are often overestimated because of inaccurate identification. Accurate data on the prevalence of the pathogenic strain(s) of E. histolytica in those regions will allow for the effective cure of patients with anti-amoebic drugs thus preventing the development of resistant types and reducing management costs.
With the advents of HIV and AIDS, several organisms have been identified as potential opportunistic pathogens. However, it is not clear whether amoebiasis is an opportunistic infection or not. Up to date, very little data has been published on the occurrence of E. histolytica in relation to HIV and AIDS. In developed countries amebiasis tends to be more common in older patients and occurs mostly among men who have sex with men or in institutions. However, in tropical regions, the epidemiology of amoebiasis is completely different and is more common among the general population and particularly among patients attending health care centers with diarrhea. Therefore, it is important to understand the epidemiology of this pathogen in tropical areas where it is responsible for most morbidity and mortality.
The recent reclassification of E. histolytica into different species now including the pathogenic Entamoeba histolytica and the non pathogenic Entamoeba dispar and Entamoeba moshkovskii has further added to the complexity of the epidemiology of amoebiasis since these three species cannot be differentiated by microscopy that is the most commonly used diagnostic method particularly in tropical countries where resources are limited, but can only be differentiated by the use of molecular methods such as the polymerase chain reaction based methodologies. Recent development of simpler but more sensitive methods such as the Loop-Mediated Isothermal Amplification (LAMP) should improve the understanding of the epidemiology of this disease.
Over the past few years we have studied the epidemiology of E. histolytica in African countries (Cameroon, Zimbabwe, and South Africa). In the present topic, we review these and other studies conducted in the African continent as well as other tropical regions in the light of new and more specific and sensitive molecular methods. The pathogenesis mechanism of amoebiasis is still not clear and recently differences in population levels of E. histolytica strains isolated from asymptomatic and symptomatic individuals have been shown to exist. One of the factors believed to be the determinant of the various clinical presentations of the disease is the organism’s virulence. The different methodologies used for the detection and epidemiology of amoebiasis will be reviewed as well as the role of E. histolytica in HIV disease. Recent advances on the pathogenesis and control of amoebiasis will also be reviewed.
Amoebiasis caused by the protozoan parasite E. histolytica was first recognized as a deadly disease by Hippocrates who described a patient with fever and dysentery (460 to 377 B.C.). With the application of a number of new molecular biology-based techniques, tremendous advances have been made in our knowledge of the diagnosis, natural history, and epidemiology of amoebiasis. Amoebiasis remains an important health problem in tropical countries where sanitation infrastructure and health are often inadequate (Ximénez et al., 2009). Clinical features of amoebiasis range from asymptomatic colonization to amoebic colitis (dysentery or diarrhea) and invasive extraintestinal amoebiasis, which is manifested most commonly in the form of liver abscesses (Fotedar et al., 2007). Current WHO estimates of 40-50 million cases of amoebic colitis and amoebic liver abscess (ALA) and up to 100,000 deaths annually, place amoebiasis second only to malaria in mortality (Stanley 2003; Ravdin 2005; WHO/PAHO/UNESCO 1997). Global statistics on the prevalence of E. histolytica infection indicates that 90% of individuals remain asymptomatic while the other 10% develop clinically overt disease (Jackson et al, 1985; Haque et al., 1999). Although all the deaths could be due to invasive E. histolytica infections, the value for the prevalence of E. histolytica is an overestimate since it dates from before the separation of the pathogen E. histolytica from the non-pathogen E. dispar (Diamond & Clark, 1993). Recently however, Entamoeba moshkovskii, a morphologically identical species, has been detected in individuals inhabiting endemic areas of amoebiasis (Ali et al., 2003, Fotedar et al., 2008, Khairnar et al., 2007, Parija & Khairnar, 2005) and could be contributing to the prevalence figures. Thus, the reclassification of E. histolytica into the three morphologically identical yet genetically different species has further added to the complexity of the epidemiology of amoebiasis since they cannot be differentiated by microscopy that is the most commonly used diagnostic method particularly in tropical countries where resources are limited. Furthermore, the worldwide prevalence of these species has not been specifically estimated. Thus, obtaining accurate species prevalence data remains a priority as there are gaps in our knowledge for many geographic regions of the tropics.
Although only a minority of E. histolytica infections - one in every four asymptomatic intestinally infected individuals – progress to development of clinical symptoms (Gathiram and Jackson, 1987; Blessmann et al., 2003; Haque et al., 2006), the exact basis for this difference remains mostly unsolved. This might be partly due to the differences in the pathogenic potential of the infecting strains (Burch et al., 1991) and/or the parasite genotype (Ali et al., 2007) or due to the variability of the host immune response against amoebic invasion (Mortimer and Chadee, 2010).
The disease mechanism and the exact prevalence and incidence of infection caused by E. histolytica are still unknown. The epidemiological data available for endemic countries however, albeit sporadic, is based mostly on the microscopic identification of the E. histolytica/E. dispar/E. moshkovskii complex, often inaccurately reported as "E. histolytica". To date many highly sensitive and specific techniques such as enzyme-linked immuno-sorbent assays (ELISA) and polymerase chain reaction (PCR) have been developed for the accurate identification and detection of E. histolytica in various clinical samples (Ackers, 2002). It is anticipated that these molecular tools will allow us to reconstruct a more reliable picture of the true epidemiology of the disease mainly in endemic regions of the world and to better our understanding of the role of the parasite and/or host factors that determine the disease outcome.
Biology of Entamoeba histolytica
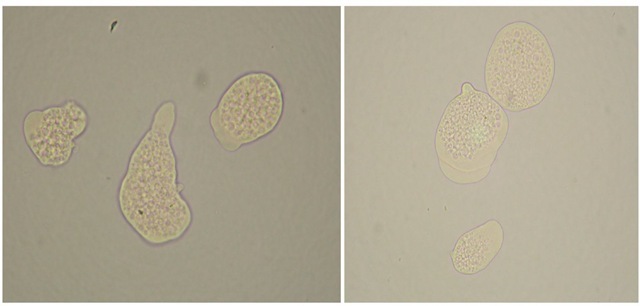
Entamoeba histolytica trophozoites (Figure 1) live and multiply indefinitely within the mucosa of the large intestine feeding normally on starches and mucous secretions and interacting metabolically with the host’s gut bacteria. However, such trophozoites commonly initiate tissue invasion when they hydrolyze mucosal cells and absorb the predigested products in order to meet their dietary provisions. Filopodia (tiny cytoplasmic extensions) that form from the surface of their trophozoites are believed to play a role in the pathogenicity of certain strains. Examples of functions related to pathogenesis include: endocytosis and/ or pinocytosis, exocytosis, tissue penetration, cytotoxic substances release or contact cytolysis of host cells. Other host factors that may also influence the invasiveness of E. histolytica are the oxidation-reduction potential and gut contents pH both of which are largely influenced by the overall nutritional state of the host.
Once the parasites invade the intestinal wall, they reach the submucosa and the underlying blood vessels. From there, trophozoites travel in the blood to sites such as the liver, lungs or skin. These parasite forms are now considered to be dead-end course since they cannot leave the host and cause infection in others. Encystation occurs in the intestinal lumen, and cyst formation is complete when four nuclei are present. These infective cysts are passed into the environment in human feces and are resistant to a variety of physical conditions. On occasions, trophozoites may exit in the stool, but they cannot survive outside the human host. The signals leading to encystations or excystation are poorly understood, but findings in the reptilian parasite Entamoeba invadens suggest that ligation of a surface galactose-binding lectin on the surface of the parasite might be the one trigger for encystations (Stanley, 2003; Eichinger, 2001). Also, several previous proteomic and transcriptomic studies have shown that a few dozens of Rab genes/proteins are involved in important biological processes, such as stress response, virulence, and pathogenesis, and stage conversion (Picazarri et al., 2008; Chatterjee et al., 2009; Novick and Zerial, 1997; Stenmark, 2009; Nozaki and Nakada-Tsukui, 2006). EhRab11A was reported to be recruited to the cell surface by iron or serum starvation, and was suggested to be involved in encystation (McGugan and Temesvari, 2003). In contrast, EhRab11B is involved in cysteine protease secretion, and its overexpression enhanced the secretion of cysteine protease (Mitra et al., 2007; Nozaki and Nakada-Tsukuia, 2006).
Fig. 1. Entamoeba histolytica trophozoites observed under the microscope stain with methylene blue (Observe that the cells did not accept the stain since they were still alive at the time the picture was taken).
The life cycle of E. histolytica is simple and consists of an infective cyst stage (10 to 15 μm in diameter) and a multiplying trophozoite stage (10 to 60 μm in diameter). Like other protozoa, E. histolytica appears incapable of de novo purine synthesis. Biochemical analysis has indicated that glutathione is not present. For this reason, E. histolytica is different from higher eukaryotes. It also uses pyrophosphate instead of ATP (McLaughlin and Aley, 1985). Mature cysts in the large intestine leave the host in large numbers and remain viable and infective in a moist, cool environment for at least 12 days. In water, cysts can live for up to 30 days. Nonetheless, they are rapidly killed by desiccation, and temperatures below 5°C and above 40°C. Mature cysts are also resistant to chlorine levels normally used to disinfect water. When swallowed, cysts pass through the stomach unharmed. In the small intestine, where conditions are alkaline and as a result of nuclear division, eight motile trophozoites are produced. These motile trophozoites then settle in the large intestine lumen, where they divide by binary fission and feed on host cells, bacteria and food particle (Figure 2). This is the first chance of the parasite making contact with the mucosa.
The organisms’ biochemistry and metabolism have been reviewed by McLaughlin and Aley (1985). It has many hydrolytic enzymes, including phosphatases, glycosidases, proteinases, and an RNAse. Major metabolic end products are carbon dioxide, ethanol and acetate. E. histolytica is more of a metabolic opportunist which is able to exploit oxygen when it is present in the environment. Glucose is metabolized via the Embden-Meyerhof pathway exclusively, and fructose phosphate is phosphorylated, prior to lysis, by enzymatic reactions unique to Entamoeba spp. Pyruvate is converted mostly to ethanol, even in the presence of oxygen, via coenzyme-A, and pyruvate oxidase. Terminal electron transfers are accomplished with ferredoxinlike iron-sulphur proteins, a trait that may contribute to the efficacy of metronidazole in treatment. Similar metabolic traits in Trichomonas vaginalis and Giardia lamblia also are metronidazole targets. Mitogen Activated Protein Kinases (MAPK) – a group of proline directed serine/threonine kinases (Bardwell, 2006) – regulate a number of different cellular processes such as proliferation, and response to a variety of environmental stresses like osmotic stress, heat shock and hypoxia (Junttila, 2008). The existence of MAPK homologues has been documented in certain parasitic protozoa. For instance ERK1 and ERK2 homologues of Giardia lamblia have been shown to play a critical role in trophozoite differentiation into cysts (Ellis et al., 2003), Pfmap2, a MAPK homologue in Plasmodium falciparum is essential for the completion of the asexual phase of the parasite lifecycle (Dorin-Semblat et al., 2006) and Leishmania major MAPK homologues exhibit an increased phosphotransferase activity in response to pH and temperature shift.On the other hand, E. histolytica has been shown to possess a single homologue of a typical MAPK gene (EhMAPK). Activation of EhMAPK in E. histolytica has been found to be associated with stress survival such as heat shock and oxidative stress response (Ghosh et al, 2010).
Fig. 2. Life cycle of E. histolytica/E. dispar. a) Mature cyst stained with 4% Lugol solution (100* magnification). b) Mature cyst without staining (100*). c) Trophozoite observed with differential interference contrast (DIC) (100*). d) Trophozoites of E. histolytica species with phagocyted erythrocytes (DIC 40*).
Epidemiology of amoebiasis and its occurrence in the era of HIV and AIDS
The epidemiology of amoebiasis around the world is complicated by the existence of three different forms that are morphological identical but genetically distinct and include E. histolytica which is a known pathogen, E. dispar and E. moshkovskii which are non pathogens (Ali et al., 2008). This is particularly relevant to the African continent as well as many other developing countries in the world, including Latin American and Asian countries, where there is lack of specific diagnostic tools. According to some studies conducted in some African countries (Alonzo et al., 1993; Molback et al., 1994; Njoya et al., 1999; Roche et al., 1999) from 6% to 75% of the population carry the parasite. These studies were conducted using microscopic examination giving a general idea on the distribution of the disease in the population. Such results require confirmation by techniques that clearly differentiate E. histolytica from E. dispar, which is not pathogenic. Countries in Central and Latin America where the parasite displays endemic behavior include Mexico, Brazil, and Ecuador. In Mexico for example, the incidence rate of intestinal amoebiasis from 1995 to 2000 was reported to be between 1000 and 5000 cases/100,000 inhabitants annually. Incidence values from 2002 to 2006 were 1128.8 to 615.85/100,000 inhabitants per year. As in other developing countries, those under 15 years of age were the most frequently affected group, with a notable increase in children aged 5-9 (Ximenez, 2009). In Aracaju, Brazil, Lawson et al (2004) demonstrated E. histolytica in 1% of cases whereas E. dispar was found in 13% of the cases. Whilst in Pernambuco state, northeastern Brazil E. dispar was found in 74.19% of culture positive samples using the PCR method, no E. histolytica was reported (Pinheiro et al., 2004). In a remote area of Ecuador, Gatti et al (2002) using isoenzyme analysis reported an 18.9% infection rate with E. histolytica while 70.3% were infected with E. dispar. In the Indian subcontinent, the prevalence of intestinal amoebiasis among hospitalized patients was found to be around 11.7% using microscopy. However, using molecular biology tools such as PCR, E. histolytica was shown to be in 3.5% of those infected (Khairnar et al., 2007). In another endemic country such as Bangladesh and using ELISA antigen detection kits, E. histolytica prevalence was found to be 4.2% among children living in the urban slums of Dhaka (Haque et al. 2006). Many studies have been conducted in different parts of the world, (Ghosh et al., 2000) but the region most concerned by this problem (Africa) remains unexplored. Thus, the epidemiology of amoebiasis still remains very uncertain particularly in this part of the world.
Following the HIV/ AIDS pandemic, numerous studies demonstrated that intestinal parasites such as Cryptosporidium sp, Microsporidia sp, Isospora belli, and Cyclospora cayetenensis were frequently associated with episodes of severe and often fatal diarrhea in both industrialized and poor countries. There have been controversies around the impact of HIV on the occurrence of amebiasis. However, recent data have shown an increase in the occurrence of E. histolytica among HIV patients in countries such as Japan, Mexico, Taiwan, and South Africa (Moran et al., 2005; Hung et al., 2008; Samie et al., 2009; Watanabe et al., 2011). With the hall mark of HIV infection being the depletion of CD4+ T cells count (below 200 cells/μ!) and the progressive decline of the mucosal immunologic defense mechanisms, HIV/AIDS patients become prone to life-threatening gastrointestinal manifestations such as diarrhea (Stark et al., 2009). Table 1 provides a summary of the prevalence studies reporting E. histolytica and/or E. histolytica/E. dispar infections in HIV positive individuals in different countries. The association of E. histolytica infections with HIV positive individuals in some studies is not clear-cut. In a Mexican study no clear association between E. histolytica and HIV has been noted. In this study, the prevalence of E. histolytica in HIV/ AIDS patients was 25.3% compared to 18.4% in a control HIV-group (Moran et al., 2005). Other studies in South American countries have shown no obvious association. However, a significant association between high levels of serum anti-E. histolytica antibodies and the presence of E. histolytica in the stool has been noted in studies from both Vietnam (Blessman et al., 2006) and Africa (Stauffer et al., 2006). In a South African study in the Vhembe district in the northern part of the country, a positive association between E. histolytica infection and HIV-positive individuals has been indicated. Among the HIV-positive individuals, those with CD4+ count less than 200 cells/μ!, were relatively more likely to be seropositive for E. histolytica (Samie et al., 2010). In a Chinese study, a higher seroprevalence of E. histolytica infections was also found in HIV-infected patients (Chen et al, 2007). Furthermore, two studies conducted in Taiwan revealed a positive association as well (Hung et al., 2005; Tsai et al., 2006).
|
Country |
Prevalence of Entamoeba species |
Reference |
|
Cuba |
1.5% (E. histolytica/dispar) |
Escobedo, A. A. 1999 |
|
Bogota, Colombia |
13% (E. histolytica) |
Florez et al., 2003 |
|
San Pedro Sula, Honduras |
5.8% (E. histolytica) |
Lindo et al., 1998 |
|
Venezuela (Zulia state) |
10.8% (E. histolytica) |
Rivero et al., 2009 |
|
Brazil |
3.3% and 1% (E. histolytica/ dispar before and after HAART) |
Bachur et al., 2008 |
|
Mexico |
25.3% in HIV+ and 18.5% in HIV-contacts (E. histolytica) |
Moran et al., 2005 |
|
Tajikistan |
25.9% (E. histolytica/dispar non HIV) |
Matthys et al., 2011 |
|
Northern India |
7.7% (E. histolytica) |
Prasad et al., 2000 |
|
Taiwan |
5.8% (E. histolytica in HIV patients) |
Hung et al., 2008 |
|
Bangladesh |
2.1% vs. 1.4% in diarrhea and control (E. histolytica) |
Haque et al., 2009 |
|
India (Kolkata) |
3.6% (E. histolytica) |
Mukherjee et al., 2010 |
|
Sydney, Australia |
3.2% (E. histolytica/E. dispar) |
Stark et al., 2007 |
|
Mazandaran province, Iran |
1.6% (E. histolytica) |
Daryani et al., 2009 |
|
Uganda |
1.4% (E. histolytica) |
Brink et al., 2002 |
|
Ethiopia |
10.3% (E. histolytica) |
Hailemariam et al., 2004 |
|
Dakar, Senegal |
5.1% (E. histolytica) |
Gassama et al., 2001 |
|
South Africa |
12.4% (E. histolytica) |
Samie et al., 2006 |
Table 1. Global prevalence of E. histolytica in HIV-infected and non-infected persons.
Over the past decade, there has been an increasingly reported risk of amebiasis in East Asian countries like Japan, Taiwan and South Korea particularly among men who have sex with men (MSM) probably due to oral-anal sexual contact (Hung et al., 2008; Watanabe et al., 2011). In Japan, E. histolytica often occur in institutions of mentally retarded individuals where outbreacks of amebiasis have been described with the prevalence rate and positive serology rate as high as 38.2% and 67.1%, respectively (Nishise et al., 2010) and has been occurring more often in HIV positive patients (Watanabe et al., 2011). In addition to HIV/AIDS, the increasing use of organ transplants and other immunosuppressed conditions such as neutropenia have been considered important risk factor for invasive amoebiasis in many countries. In Colombia for example, a study of organ transplant patients revealed that about 24.7% had detectable antiamoebic antibodies (Reyes et al., 2006) whereas in another study 14.3% neutropenic patients were found to have antiamoebic antibodies (Cardona et al., 2004).
Certain risk behaviors, such as homosexual relations and practicing oro-anal sex, can exacerbate the possibility of acquiring E. histolytica infections as well as other intestinal parasites such as Cryptosporidium spp., where the symptomatic pictures are more severe than those of immunocompetent individuals (Tatiana et al., 2008; Hung 2008). A recent study in Vietnam had indicated that socio-economic and personal hygiene factors determined infection with E. histolytica, rather than exposure to human and animal excreta in agricultural activities (Pham duc et al., 2011). In a study in Bangladesh, it was shown that wet environment is not the only factor that affects the detection curve of E. histolytica, but anti-Carbohydrate Recognition Domain IgA level in the gut is another determining factor for its occurrence in a closed population (Haque et al., 2006). Although, numerous seroprevalence studies suggest that HIV/ AIDS individuals are at a higher risk of E. histolytica infections and are therefore more likely to develop symptomatic infections or severe forms of the disease, modest data exist to support these findings and further research is needed to confirm this hypothesis.
Diagnosis of amoebiasis
Amoebiasis diagnosis rests on the demonstration of E. histolytica trophozoites or cysts in stool or colonic mucosa of patients. For many years a direct smear examined either as a wet mount or fixed and stained was done by microscopic examination of stool. Repeated stool sample examinations (at least three) may be needed. The presence of haematophagous amoebic trophozoites in a stool sample has always suggested E. histolytica infections (Gonzalez-Ruiz, A. et al 1994). Nonetheless, the specificity of this finding was further reduced when it was demonstrated that in some patients E. dispar also contains RBCs (Fotedar et al., 2007). Also, in view of the high frequency of E. dispar in many areas, dysentery due to entities such as shigellosis and campylobacter will probably be misdiagnosed as amoebic colitis if microscopy is the sole diagnostic criteria (Stanley 2003). However, in the absence of haematophagous trophozoites, the sensitivity of microscopy is limited by its ability to distinguish between samples infected with E. histolytica and the morphologically identical E. dispar and E. moshkovskii. Confusion between E. histolytica, other non-pathogenic amoeba and white blood cells such as macrophages and polymorphonuclear cells in feces frequently result in the overdiagnosis of amoebiasis. Delays in the processing of stool samples affect the sensitivity of light microscopy, which under the best circumstances is only 60% of that of the stool culture method followed by isoenzyme analysis (Krogstad et al., 1978).
Stool culture technique followed by isoenzyme analysis has been considered as the "gold standard" for many years. This method has been used to distinguish between E. histolytica and E. dispar. For more details on the culture technique the reader is advised to consult reference (Clark and Diamond, 2002). Culture of E. histolytica can be performed from fecal specimens, rectal biopsy specimens, or liver abscess aspirates. However, the process usually takes between 1-4 weeks to perform and requires sophisticated laboratory equipment making it not feasible as a routine procedure especially in the developing world where E. histolytica is rampant. The rate of success of E. histolytica culture in reference laboratories has been reported to be between 50 and 70%. Moreover, isoenzyme (zymodeme) analysis is labor intensive, costly and often produces false-negative results for many microscopy positive stool specimens (Strachan et al., 1988).
Serological methods may be useful diagnostically to detect infections with E. histolytica in developed countries where infections are not as common as in endemic developing nations (Ohnishi et al., 1997). In developing countries individuals are constantly exposed to E. histolytica making serological tests unable to definitively distinguish past from current infections (Caballero et al., 1994). Amoebic serology is highly sensitive and specific for the diagnosis of ALA (Zengzhu et al., 1999). Conversely, a study of asymptomatic individuals living in an E. histolytica endemic area of Vietnam revealed that about 83% of those infected had detectable anti-amoebic antibodies (Blessmann et al., 2002). Several assays for the detection of antibodies to E. histolytica infections have been developed (Table 2). These include: indirect hemagglutination (IHA), latex agglutination, immunoelectrophoresis, counterimmunoelectrophoresis (CIE), the amebic gel diffusion test, immunodiffusion, complement fixation, indirect immunofluorescence assay (IFA), and enzyme-linked immunosorbent assay (ELISA). With the exception of ELISA, all the other tests have been either costly to perform (Complement fixation), less sensitive and nonspecific (IHA and Latex agglutination test), time consuming (immunodiffusion) or requires skills in culture and antigen preparation (IFA) (Fotedar et al., 2007).
|
Serological Assay |
Sensitivity (%) |
Specificity (%) |
Reference(s) |
|
IHA |
100a, 99 |
90.9-100a , 99.8 |
Pillai et al., 1999; Hira et al., 2001 |
|
Novagnost Entamoeba IgG |
>95 |
>95 |
Manufacturer’s recommendation |
|
I.H.A. Amoebiasis |
93 |
97.5 |
Robert et al., 1990 |
|
Amebiasis Serology microplate ELISA |
95 |
97 |
Manufacturer’s recommendation |
|
RIDASCREEN Entamoeba (IgG detection) |
100, 97.7-100 (100) |
95.6, 97.4 (100) |
Manufacturer’s recommendation; Knappik et al., 2005 |
Table 2. List of some of the commercially available antibody assays used for the diagnosis of amoebiasis.
ELISA is a reliable, easy to perform and rapid method for the diagnosis of E. histolytica infections especially in developing countries. It has been used widely for the study of the epidemiology and diagnosis of symptomatic amoebiasis (intestinal and/or extraintestinal). An ELISA to detect antibodies to E. histolytica has been shown to be 97.9% sensitive and 94.8% specific for detection of E. histolytica antibodies in ALA patients in a non endemic country (Hira et al., 2001). Unlike IgG, immunoglobulin M (IgM) is short lived and does not remain in the serum for longer periods making it a very useful marker for the detection of present or current E. histolytica infections. An ELISA for the detection of serum IgM antibodies to the amoebic Gal or GalNAc-inhibitable adherence lectin has been reported. In this study, conducted in Egypt, anti-lectin IgM antibodies in the serum were detected in 45% of patients who had been suffering from acute colitis for <1 week (Abd-Alla et al., 1998). Since there is no cross-reaction with other non-E. histolytica parasites (Goncalves et al., 2004), the use of ELISA thus seems to be an excellent choice for the routine laboratory diagnosis as well as the surveillance and control of amoebiasis in the developing world. The newer methods available to distinguish between E. dispar and E. histolytica have thrown into question the commonly accepted figure of 500 million infections worldwide suggesting that the actual number may be closer to 50 million. PCR and monoclonal antibody techniques are now available to distinguish between these three species in fresh and preserved stool samples, including those with mixed infections. Several investigators have developed ELISAs that detect antigens in fresh stool samples with sensitivity closer to that of stool culture methods and PCR. These ELISAs are usually easy and rapid to perform. Copro-antigen based ELISA kits specific for E. histolytica exploit monoclonal antibodies against the Gal/ GalNAc-specific lectin of E. histolytica (E. histolytica II; TechLab, Blacksburg, VA) or against serine-rich antigen of E. histolytica (Optimum S kit; Merlin Diagnostika, Bornheim-Hersel, Germany). Other ELISA kits include the Entamoeba CELISA PATH kit (Cellabs, Brookvale, Australia) and the ProSpecT EIA (Remel Inc.; previously manufactured by Alexon-Trend, Inc., Sunnyvale, CA) (Fotedar et al., 2007). The early nineties of the 20th century have witnessed the introduction by TechLab of an ELISA kit for the specific detection of E. histolytica in feces. This antigen detection test captures and detects the parasite’s Gal/GalNAc lectin in stool samples. It can also be used for the detection of the lectin antigen in the serum and liver abscesses in patients with invasive intestinal amoebiasis and ALA (Haque et al., 2000). However, the diagnosis of ALA normally relies on the identification of liver lesions and positive anti-E. histolytica serology. Yet neither provides conclusive results for ALA. The Gal/GalNAc lectin is conserved and highly immunogenic, and because of the epitopic differences in the lectins of E. histolytica and E. dispar, the test enables specific identification E. histolytica (Haque et al., 1993; Mirelman 1997). Because of some disadvantages observed with the TechLab ELISA kit, a newer more sensitive and specific version, TechLab E. histolytica II kit, was produced. This second -generation E. histolytica II kit has demonstrated good sensitivities and specificities when compared to real-time PCR (71 to 79% and 96 to 100%, respectively) (Roy et al., 2005; Visser et al., 2006). Other studies however, have reported a lesser sensitivity (14.3%) and specificity (98.4%) in comparison to stool culture and isoenzyme analysis (Gatti et al., 2002). Cross reactivity is another concern with the use of the assay, since it seems that E. dispar positive samples by means of PCR may sometimes give false-positive outcomes (Furrows et al., 2004). Accordingly, accurate detection of E. histolytica, E. dispar and E. moshkovskii could be helpful for diagnostic and epidemiological studies in places where it is impractical and expensive to use molecular assays and where amoebiasis is most prevalent, such as in the developing countries. An antigen detection kit for the specific identification of E. dispar and E. moshkovskii is yet to be developed.
Several PCR-based techniques that amplify and detect E. histolytica DNA are currently used for the clinical and epidemiological studies in non-endemic rich countries (Acuna-Soto et al., 1993; Katzwinkel-Wladarsch et al., 1994; Calderaro et al., 2006; Hamzah et al., 2006). The sensitivity and specificity of PCR-based methods for the diagnosis of E. histolytica infection approach those of stool culture followed by isoenzyme analysis. PCR methods can be used to detect E. histolytica in stool, tissues and liver lesion aspirates. Of all the different gene targets used to identify E. histolytica, the small-subunit rRNA gene (18SrDNA) is believed to be more sensitive than the best antigen detection method used and performs equally well compared to stool culture (Mirelman et al., 1997).
Several groups have developed a variety of excellent conventional PCR assays, targeting different genes, for the direct detection and differentiation of E. histolytica, E. dispar, and E. moshkovskii DNA in clinical specimens such as stool and liver abscess samples (Tanyuksel and Petri Jr., 2003; Paul et al., 2007). Of all the targeted genes, assays amplifying the 18SrDNA genes are the ones in wide use as they are present in multiple copies on extrachromosomal plasmids thus making them easily detectable than single copy genes (Battacharya et al., 1989). Other gene targets used in PCR to study the epidemiology of E. histolytica include: the serine-rich E. histolytica protein (SREPH) gene (Stanley et al., 1990), cysteine proteinases gene and actin genes (Freitas et al., 2004). The SREHP is also used to study the genotypes of E. histolytica in human populations. However, it is now being replaced by the use of PCR amplification of tRNA gene-linked short tandem repeats which in addition to providing details of the epidemiology of E. histolytica, it also provides a tool to predict the outcome of the infection (Ali et al., 2005).
A nested multiplex PCR was developed by many groups. This method has the added advantage of increasing the sensitivity and specificity of the test whilst simultaneously detecting and differentiating E. histolytica and E. dispar from DNA extracted from microscopy-positive stool specimens (Evangelopoulos et al., 2000; Hung et al., 2005; Nunez et al., 2001). A nested PCR method for the identification of E. moshkovskii in fecal samples was developed as a nested 18S rDNA PCR followed by restriction endonuclease digestion (Ali et al., 2003). The method exhibited a high sensitivity and specificity (100%). Real time PCR is another type of PCR which is more sensitive than the conventional PCR. It is faster than the conventional PCR and characterized by the elimination of gel analysis and other post-PCR analysis, thus reducing the risk of contamination and cost (Klein 2002). However, its application in developing countries is limited to research only. Real-time PCR allows specific detection of the PCR product by binding to one or two fluorescence-labeled probes during PCR, thereby enabling continuous monitoring of the PCR product formation throughout the reaction. Furthermore, real-time PCR is a quantitative method and allows the determination of the number of parasites in various samples (Fotedar et al., 2007). Despite being used for the successful identification of E. histolytica, E. dispar and E. moshkovskii, the various PCR methods use is still confined to research institutes in the developing world where amoebiasis is endemic. PCR-based methods application in routine clinical diagnostic laboratories in low income societies is hindered by difficulties such as cost, and time to perform the test.
A new platform for the detection of pathogens has been developed known as loop-mediated isothermal amplification (LAMP) and was developed in 2000 by Notomi and colleagues. This method uses a set of two specifically designed inner primers and two outer primers that recognize six distinct regions of the targeted DNA. The reaction is performed under isothermal conditions and simple incubators, such as a water bath or heat block, are adequate for the specific amplification of the desired genetic material. Considering these advantages, the LAMP assay could be a useful and valuable diagnostic tool particularly in developing countries where most of the infections are common as well as in hospital laboratories. Recently this method was developed specifically for the detection of E. histolytica (Liang et al., 2009). The efficiency of the developed method was compared to that of existing PCR methodology and was similar in terms of sensitivity and specificity. This method needs further evaluations to be used in local conditions in Africa in order to improve the understanding of amebiasis in the continent as well as elsewhere.