Introduction
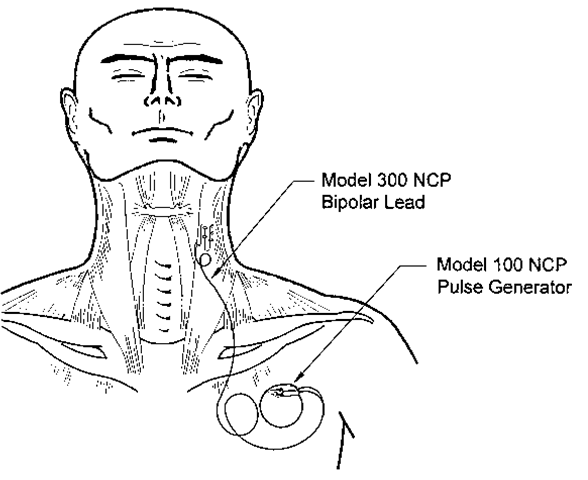
Vagus nerve stimulation (VNS) by the implantable neurocybernetic prosthesis (NCP) from Cyberonics, Inc. is emerging as a novel adjunct in the management of patients with medically refractory seizures. This device delivers intermittent electrical stimulation to the left cervical vagus nerve trunk, which secondarily transmits rostral impulses to exert widespread effects on neuronal excitability throughout the central nervous system (Fig. 1). We have comprehensively reviewed the theoretical rationale, practical background, and clinical application of VNS in previous publications [1-3]. The operative procedure for implanting the NCP device has also been presented in detail elsewhere [4-6]. This topic summarizes pragmatic issues pertaining to VNS, such as patient selection and surgical technique.
Clinical Utility of VNS
Review of Safety and Efficacy
Clinical experience with vagus nerve stimulation began in 1988 with the first human implantation of the NCP system. Since then, more than 1,000 patients have participated in seven clinical trials throughout 26 countries, and more than 3000 patient-years of data have accrued. These studies confirm the long-term safety, efficacy, feasibility, and tolerability of VNS, as well as the durability of the NCP device [1,7].
Figure 1 Schematic representation of VNS therapy. A pulse generator inserted in the subcutaneous tissues of the upper left chest delivers intermittent electrical stimulation to the cervical vague nerve trunk via a bifurcated helical lead.
Vagus nerve stimulation gained approval by the United States Food and Drug Administration (FDA) in 1997. Post-marketing experience with more than 7,000 patients validates the earlier clinical trials, and in 1999, the Therapeutics and Technology subcommittee of the American Association of Neurology declared VNS "effective and safe, based on a preponderance of class I evidence” [8]. Although VNS requires a large initial investment because of the price of the device itself as well as its surgical insertion, cost-benefit analysis suggests that the expense of VNS is recovered within 2 years of follow-up [9].
Recently, a meta-analysis was performed of the 454 patients enrolled in one of five multicenter clinical trials conducted in the United States before FDA approval [7]. For the study population as a whole, the median reduction in seizure frequency was 35% at 1 year, 43% at 2 years, and 44% at 3 years. An important observation is that the response to VNS is maintained during prolonged stimulation, and unlike chronic medication therapy, seizure control actually improves with time.
The response of individual patients to VNS varies widely. Although 1% to 2% of subjects enjoy complete seizure cessation, others derive no benefit whatsoever. The remainder experience intermediate results. In the collective study experience, the proportion of patients who sustained a 50% reduction in baseline seizure frequency was approximately 23% at 3 months and 43% after 2 years. These improvements occurred in a highly refractory population of patients who typically had an average of 1.7 seizures per day despite administration of more than two antiepileptic medications.
In spite of the well-known functions of the vagus nerve as the principal efferent component of the parasympathetic nervous system, VNS has not been shown to adversely affect any aspect of physiological function, including cardiac rhythm, pulmonary function, gastrointestinal motility, and secretion. Unlike many antiepileptic medications, VNS therapy does not impair cognition, balance, or emotion. Plasma concentrations of antiepileptic medications remain unchanged.
Side effects are typically transient, mild, and limited to cycles of stimulation. Initially, patients may experience voice alteration (20%-30%), paresthesias (10%), or cough (6%), but the incidence of these adverse effects diminishes greatly over time. Surgical complications are rare, and device failures are also uncommon.
Indications and Contraindications
The selection criteria for insertion of the NCP system remain in evolution and reflect current governmental standards, as well as institutional biases and general guidelines from prior clinical trials [1,2]. Currently, the device is only approved by the FDA "as an adjunctive therapy in reducing the frequency of seizures in adults and adolescents over 12 years of age with partial onset seizures which are refractory to antiepileptic medications” [1]. However, favorable results have been obtained with off-label use among children as young as age 3 and among patients with Lennox-Gastaut or other primarily generalized seizures syndromes [10]. Preliminary experience with infantile spasms has been disappointing, however. Patients with both idio-pathic epilepsy and seizures of structural etiology are considered appropriate candidates. Of note, VNS has been used successfully among patients who have failed previous surgical procedures, confirming the potential efficacy of VNS in highly refractory patient populations.
The definition of medical intractability varies from center to center. Standards from previous studies commonly required a frequency of at least six seizures per month and a seizure-free interval of no longer than 2 to 3 weeks despite therapy with multiple medications [1,2]. However, seizure frequency, seizure type, severity of attacks, drug toxicity, and overall impact on quality of life must all be considered before a patient is deemed refractory to pharmacotherapy. Adequate monitoring of patient compliance and sufficient trials of investigational drugs must also be assured.
As noted above, the response to VNS is highly variable, and previous clinical trials have failed to characterize the demographic factors that predict a favorable outcome [1,2]. Furthermore, VNS is rarely curative. Therefore, at present, we do not consider the NCP device an alternative to conventional methods of epilepsy surgery that offer a higher likelihood of seizure cessation, and we generally reserve VNS for patients in whom such operations are not indicated. These include those patients whose seizure focus is bilateral, not associated with a structural abnormality, or cannot be completely resected because of overlap with functional cortex.
The NCP system cannot be inserted in patients who have undergone a prior left cervical vagotomy. Furthermore, the safety of VNS has not been tested in several conditions in which impairment of vagus nerve function might produce deleterious effects. Thus, relative contraindications include progressive neurological or systemic diseases, pregnancy, cardiac arrhythmia, asthma, chronic obstructive pulmonary disease, active peptic ulcer disease, and insulin-dependent diabetes mellitus [1].
Practical Background
Anatomy and Physiology of the Vagus Nerve
The vagus nerve is generally regarded as an efferent projection that innervates the larynx and provides parasympathetic control of the body; however, the majority of its fibers are special visceral and general somatic afferents leading towards the brain [1]. The latter originate from receptors in the viscera and terminate in diffuse areas of the central nervous system, many of which are potential sites of epileptogenesis. These include the cerebellum, diencephalon, amygdala, hippocampus, insular cortex, and multiple brain-stem centers. Some of these projections relay through the nucleus tractus solitarius, whereas others form direct, monosynaptic connections with their targets. It remains unclear which of these pathways underlies the mechanism of VNS action, but the locus coeruleus and raphe nucleus appear to be key intermediaries, as bilateral lesions of these centers abolish the seizure-suppressing effects of VNS therapy in animal models [2].
Several branches of the vagus nerve arise cephalad to the midcervical trunk, where the VNS electrodes are applied [5]. These include projections to the pharynx and carotid sinus, as well as superior and inferior cervical cardiac branches leading to the cardiac plexus. Both the right and left vagus nerves carry cardiac efferent fibers, but anatomical studies in dogs suggest that those on the right side preferentially supply the SA node of the heart, whereas those on the left side preferentially innervate the AV node. For this reason, the NCP system is generally inserted on the left side. Nevertheless, stimulation of the left vagus nerve rarely may cause bradycardia or asystole, even at FDA-approved settings.
As mentioned, the NCP device is generally applied to the midcervical portion of the vagus nerve trunk, distal to the origin of the superior and inferior cervical cardiac branches; this may represent another reason why the incidence of bradycardia is low [5]. Nonetheless, the diameter, appearance, and location of the cardiac branches may approximate those of the nerve trunk itself, and care must be taken to avoid mistaking the two. If the cardiac branches are stimulated directly, small currents as low as 0.8 mA may produce significant bradycardia [5].
The midcervical portion of the vagus nerve is relatively free of branches [5]. The superior laryngeal nerve arises rostral to the carotid bifurcation before descending toward the larynx, and high currents applied to the midcervical nerve trunk may recruit these fibers, leading to tightness or pain in the pharynx or larynx. The recurrent laryngeal nerve travels with the main trunk and branches caudally at the level of the aortic arch before ascending in the tracheo-esophageal groove. As a result, hoarseness is a common occurrence during periods of stimulation or after VNS implantation.
Regional Anatomy of the Carotid Sheath
In addition to branches of the vagus nerve trunk, several other nerves in the vicinity of the carotid sheath risk hazard from the implantation procedure itself or from subsequent stimulation. The hypoglossal nerve arises cephalad to the midcervical region, making unilateral tongue weakness an infrequent complication of VNS implantation. The phrenic nerve lies deep to a fascial plane beneath the carotid sheath, and hemiparalysis of the diaphragm has been reported with stimulation at high output currents, although not as an operative complication.
The sympathetic trunk lies deep and medial to the common carotid artery. It gives off fibers that ascend with the internal carotid artery (ICA) toward the intracranial contents. We are aware of one case of Horner’s syndrome after insertion of the VNS device, caused either by manipulation of the sympathetic plexus itself or by traction on the sympathetic fibers around the ICA.
Weakness in the muscles of the lower face may result from injury to branches of the facial nerve, which ramify through the caudal aspect of the parotid gland. In general, hypoglossal and facial nerve injury are more common sequelae of carotid endarterectomy incisions, which tend to be higher than those used for placement of the VNS device.
NCP Device Components
Figure 1 depicts a schematic representation of VNS therapy. A pulse generator inserted in the subcutaneous tissues of the upper left side of the chest delivers intermittent electrical stimulation to the cervical vagus nerve trunk through a bifurcated helical lead.
In addition to the implantable lead and pulse generator, the NCP system includes a number of peripheral components, such as a telemetry wand that interrogates and programs the pulse generator noninvasively. This programming wand is powered by two 9V batteries and is interfaced with an IBM-compatible computer that runs a menu-based software package furnished by Cyberonics. The system also includes a hand-held magnet that patients may carry with them to alter the character of stimulation that the generator delivers.
The pulse generator has approximately the same size and shape as a cardiac pacemaker. It contains an epoxy resin header with receptacles that accept the connector pins extending from the bifurcated lead. The generator is powered by a single lithium battery encased in a hermetically sealed titanium module. Under normal conditions, the generator has a projected battery life of approximately 6 to 8 years. Once it has expired, the generator can be replaced with the patient under local anesthesia during a simple outpatient procedure.
The generator contains an internal antenna that receives radiofrequency signals emitted from the telemetry wand and transfers them to a microprocessor that regulates the electrical output of the pulse generator. The generator delivers a charge-balanced waveform characterized by five programmable parameters: output current, signal frequency, pulse width, signal on-time, and signal off-time. These variables are titrated empirically in the outpatient setting, according to individual patient tolerance and seizure frequency. Altering the parameters of stimulation will have various consequences on VNS efficacy, side effects, and battery life.
The generator has two accessories. One is a hairpin-shaped resistor used during preliminary electrodiagnostic testing before implantation, to test the internal impedance of the generator. The other is a hexagonal torque wrench used to tighten the set screws that secure the lead connector pins to the epoxy resin header of the generator.
The generator is still contained within its package, but it can be interrogated by the telemetry wand. The generator must pass this system check before it is opened onto the sterile field. The failure rate of the generator is extremely low, but it is recommended that a backup generator be available in the operating room at all times.
The bipolar lead is insulated by a silicone elastomer, and can thus be safely implanted in patients with latex allergies. One end of the lead contains a pair of connector pins that inserts directly into the generator, whereas the opposite end contains an electrode array consisting of three discrete helical coils that wrap around the vagus nerve. The middle and distal coils represent the positive and negative electrodes, respectively, whereas the most proximal one serves as an integral anchoring tether that prevents excessive force from being transmitted to the electrodes when the patient turns his neck. The leads come in two sizes, measured by the internal diameter of each helix. The majority of patients can be fitted with the 2-mm coil; however, it is desirable to have the 3 mm one available in the operating room as well.
Each electrode helix contains three loops. Embedded inside the middle turn is a platinum ribbon coil that is welded to the lead wire. This shape permits the platinum ribbon to maintain optimum mechanical contact with the nerve. Suture tails extending from either end of the helix permit manipulation of the coils without injuring these platinum contacts. Damage to the vagus nerve itself is greatly reduced by the self-sizing, open helical design of the NCP electrode array, which permits body fluid interchange with the nerve. Thus, compared with cuff electrodes, mechanical trauma and ischemia to the nerve are minimized. The electrode is intended to fit snugly around the nerve while avoiding compression, thus allowing the electrode to move with the nerve and minimizing abrasion from relative movement of the nerve against the electrode.
The hand-held magnet performs several functions. When briefly passed across the chest pocket where the generator resides, it manually triggers a train of stimulation superimposed on the baseline output. Such on-demand stimulation can be initiated by the patient or a companion at the onset of an aura, in an effort to diminish or even abort an impending seizure. The parameters of this magnet-induced stimulation may differ from those of the prescheduled activation. Alternatively, if the device appears to be malfunctioning, or if the patient wishes to terminate all stimulation for any other reason, the system can be indefinitely inactivated by applying the magnet over the generator site continuously. Finally, patients are instructed to test the function of their device periodically by performing magnet-induced activation and verifying that stimulation occurs. Most patients can perceive the stimulation as a slight tingling sensation in the throat.
Surgical Considerations
Operative Procedure
Insertion of the NCP device takes less than 2 hours and is typically performed under general anesthesia, thus minimizing the possibility that an intraoperative seizure might compromise the surgery. However, regional cervical blocks have also been used in awake patients. It can be performed as an outpatient procedure, but it may be desirable to observe patients overnight for vocal cord dysfunction, dysphagia, respiratory compromise, or seizures induced by anesthesia, even though these complications are rare. Prophylactic antibiotics are administered preoperatively and maintained for 24 hours postoperatively.
The patient is positioned supine with a shoulder roll beneath the scapulae to provide mild neck extension. This facilitates passage of the tunneling tool that connects separate incisions in the neck and chest. The head is rotated 30 to 45 degrees toward the right, bringing the left sternocleido-mastoid muscle into prominence.
Many options exist for placement of the skin incisions. Often, a 5-cm transverse chest incision is made approximately 8 cm below the clavicle, centered above the nipple. The underlying fat is dissected to the level of the pectoralis fascia, and a subcutaneous pocket is fashioned superiorly. Others have suggested a deltopectoral incision with inferior dissection to create the pocket, but we believe that the scar tissue formed beneath the pectoral incision helps prevent caudal migration of the generator. Recently, we have been using a lateral incision along the anterior fold of the axilla, which affords better cosmetic results, especially among women.
Next, a 5-cm longitudinal incision is made along the anterior border of the sternocleidomastoid muscle, centered over its midpoint. Generally, this incision is a little lower than that for an endarterectomy. Alternatively, a transverse skin incision at C5-6, similar to the approach for an anterior cervical discectomy, can be made. For the inexperienced surgeon, the longitudinal incision permits a wider exposure, which facilitates electrode placement through this aperture.
The platysma muscle is divided vertically, and the investing layer of deep cervical fascia is opened along the anterior border of the sternoclei-domastoid, allowing it to be mobilized laterally. After palpation of the carotid pulse, the neurovascular bundle is identified and sharply incised to reveal its contents. Self-retaining retractors with blunt blades expedite this stage of the procedure. Care is taken to limit the exposure between the omohyoid muscle and the common facial vein complex, thus minimizing potential hazard to adjacent neurovascular structures.
Within the carotid sheath at the level of the thyroid cartilage, the vagus nerve is generally encountered deep and medial to the internal jugular vein, encased in firm areolar tissue lateral to the common carotid artery. Great variability exists in the relative position of these structures, however, and the strategy by which the nerve is isolated from the remainder of the neurovascular bundle must account for such individual diversity. We attempt to minimize direct manipulation of the nerve itself. Instead, we prefer to mobilize the vessels away from the nerve. Dissection generally commences with isolation and retraction of the internal jugular vein using vessel loops.
Next, the nerve trunk is identified and dissected with the aid of the operating microscope or surgical loupes. At least 3 to 4 cm of the nerve must be completely freed from its surrounding tissues. At this stage, we have found that the insertion of a blue background plastic sheet between the nerve and the underlying vessels greatly facilitates the subsequent steps of the procedure. The technique of mobilizing the vessels away from the nerve usually preserves the vasa nervosum. This nuance may reduce the incidence of postoperative complications, such as hoarseness.
A tunneling tool is then used to create a subcutaneous tract between the two incisions. The tool is directed from the cervical to pectoral sites, to minimize potential injury to the vascular structures of the neck.
Depending on the relative size of the exposed nerve, either a small or large helical electrode is then selected for insertion. The lead connector pins are passed through the tunnel and emerge from the chest incision, whereas the helical electrodes remain exposed in the cervical region. Before applying the electrodes, the lead wire should be directed parallel and lateral to the nerve, with the coils occupying the gap between them.
Each coil is applied by grasping the suture tail at either end and stretching the coil until its convolutions are eliminated. The central turn of this unfurled coil is applied either obliquely or perpendicularly across or beneath the vagus trunk and wrapped around the surface of the nerve. The coil is then redirected parallel to the nerve as the remainder of its loops are applied proximal and distal to this midpoint (Fig. 2). The memory within the elongated coil will cause it to reassume its helical configuration and conform to the nerve snugly.
While all these maneuvers are taking place, additional electrodiagnos-tic testing of the generator is simultaneously carried out between the neurology team and the scrub technician. With the hairpin resistor inserted into the receptacles for the lead connector pins, the telemetry wand interrogates the device from within a sterile sheath to measure its internal impedance. Once the generator passes this preimplant diagnostic test, it is ready for insertion.
Figure 2 Technique of helical electrode placement. A, Each coil is applied by grasping the suture tail at either end and stretching the coil until its convolutions are eliminated. B, The central turn of this unfurled coil is applied either obliquely or perpendicularly across or beneath the vagus trunk and wrapped around the surface of the nerve. C, The coil is then redirected parallel to the nerve as the remainder of its loops are applied proximal and distal to this midpoint.
The lead connector pins are connected to the pulse generator and secured to their receptacles with set screws, using the hexagonal torque wrench. It is important to completely insert the hex wrench into its socket in the epoxy header, to decompress the backpressure that builds up as the connector pins enter the receptacles. This step is essential to form a good contact between the lead and the generator. If the connector pins fail to make such contact, the generator may attempt to overcome the resulting increased impedance by augmenting the output current, leading to intermittent symptoms of overstimulation.
Additional electrodiagnostic examination is then performed to appraise the coupling of all connections and to verify the integrity of the overall system. Then, a 1-minute lead test is performed at a frequency of 20 Hz with an output current of 1 mA and a pulse width of 500 microseconds. During this test stimulation, the responses of the patient’s vital signs and electrocardiogram are monitored. Rarely, profound bradycardia will result, necessitating the use of atropine. The incidence of this event is thought to be less than 1 in 1000 [11]. If it occurs, attention should be directed to the lead to assure that the electrodes encircle the vagus nerve trunk itself, rather than one of its cardiac branches [5]. After the test stimulation, the generator is restored to its inactive status until 1 to 2 weeks postoperatively. This waiting period allows for resolution of postoperative edema and proper fixation of the electrode to the nerve.
The redundant portion of the lead between the generator and electrode is secured to several areas of the cervical fascia with Silastic tie-downs. The objective is to form superficial and deep-restraint configurations that help prevent excessive traction from being transmitted to the electrodes during repetitive neck motion. First, a U-shaped strain relief bend is made inferior to the anchoring tether, and the distal lead is secured to the fascia of the carotid sheath. Next, a strain relief loop is established by securing the lead to the superficial cervical fascia between the sternocleidomastoid and platysma muscles. Care is taken not to sew the lead directly to the muscle.
Finally, the generator is retracted into the subcutaneous pocket and secured to the pectoralis fascia with O-Prolene or similar nonabsorbable suture, using the suture hole contained within the epoxy resin header. Any excess lead is positioned in a separate pocket at the side of the generator. To prevent abrasion of the lead, however, it should not be placed behind the pulse generator. Wound closure then proceeds in standard multilayer fashion, using a subcuticular stitch for the skin. The cosmetic results are generally very good.
Lead Removal or Revision
In some circumstances, it may become necessary to remove or replace the electrodes that encircle the vagus nerve trunk. Fibrosis and adhesions may develop in the vicinity of the vagus nerve; however, Espinosa [12] has demonstrated that the spiral electrodes may be safely removed from the nerve, even years after they were implanted.
Complication Avoidance and Management
In the meta-analysis mentioned above [7], the most commonly observed surgical complication was infection of either the generator site or lead implantation site. The overall infection rate was 2.86%, but more than half these patients were successfully treated with antibiotic therapy alone, whereas only about 1.1% required explantation of the device.
Transient vocal cord paralysis is the second most common surgical complication of VNS implantation. The incidence of this event in the collective study experience was only 0.7%. However, because video strobos-copy and formal swallowing assessments are rarely performed after surgery, it is possible that more cases went undetected, and the true prevalence of vocal cord paresis is poorly understood [5]. Fortunately, most reported cases resolve clinically.
Temporary lower facial hypesthesia or paralysis occurred in another 0.7% of patients in the meta-analysis. As stated above, excessively high surgical incisions could have been a cause.
To date, of more than 7,000 implantation procedures, only four cases of intraoperative bradycardia or asystole have been reported during the lead test, accounting for an incidence of less than 0.1%. Asconape [11] has analyzed the factors that potentially contribute to this event and the means of their prevention. As mentioned, the superior or inferior cervical cardiac branches might be mistaken for the vagus trunk itself, and correct positioning of the electrodes on the intended nerve must be verified. Proper placement of the skin incision, centered over the midcervical portion of the nerve, will also help avert this complication. Current spread to the cardiac nerves can be minimized by measures that insulate them from the midcervical trunk during the lead test, such as placement of a Silastic dam beneath the nerve trunk and removal of pooled blood or saline from the vicinity. Finally, the current should be ramped up in small increments during the lead test, starting with 0.25 mA.
As stated above, we have found it preferable to mobilize the vascular structures away from the nerve trunk, thus minimizing direct manipulation of the nerve itself. We believe that this practice may improve the efficacy of subsequent stimulation and diminish the incidence of surgical complications, such as hoarseness. Other precepts of good surgical technique, gained from experience and familiarity with the implantation procedure, will also contribute to improved outcomes.