Pain
Pain is the perception of an unpleasant sensation. Painful (noxious) stimuli (e.g., sharp pricking or slow burning) stimulate specialized receptors called nociceptors. The reception of signals from nociceptors by the central nervous system (CNS) is called nociception. Different components of the pathway mediating nociception are described in the following sections.
Nociceptors. Nociceptors are free nerve endings. There are three types of receptors activated by different noxious stimuli, and they are listed in Table 15-3 according to their functional properties. Mechanical nociceptors are activated by mechanical stimuli (e.g., sharp pricking); thermal and mechano-thermal receptors are activated by stimuli that cause slow, burning pain; and polymodal receptors are activated by mechanical stimuli as well as temperature (e.g., hot or cold burning sensation).
TABLE 15-3 Types of Nociceptors
|
Receptor Type |
Fiber Group |
Sensation |
|
Mechanical |
AS |
Sharp, pricking |
|
Thermal and |
AS |
Slow burning, cold sharp, |
|
mechano-thermal |
pricking |
|
|
Polymodal |
C |
Hot, burning sensation, cold, and mechanical stimuli |
Afferents Carrying Pain Sensations. Information regarding fast and acute pain sensations is conducted to the CNS by small, myelinated AS fibers; conduction velocity in these fibers is much faster than that of C fibers. Slow, chronic pain sensation is carried to the CNS by unmyelinated C fibers. Both types of fibers enter the spinal cord at the apex of the dorsal horn, branch, and then ascend and descend for one to three segments to enter the dorsal horn. Anatomical Pathways Mediating Pain Sensations From the Body. The cell bodies of sensory neurons mediating pain are located in the dorsal root ganglia (first-order neurons). The noci-ceptors represent nerve endings of the peripheral axons of the sensory neurons located in the dorsal root ganglia. The central axons (both AS and C fibers) of these sensory neurons reach the dorsal horn and branch into ascending and descending collaterals, forming the dorsolateral tract (fasciculus) of Lissauer. In Lissauer’s tract, these fibers (AS and C fibers) ascend or descend a few spinal segments, enter the gray matter of the dorsal horn, and synapse on neurons located in laminae I and II (substantia gelati-nosa). Sensory information from laminae I and II is transmitted to second-order neurons located in laminae IV to VI. The second-order neurons in laminae IV to VI are collectively called the nucleus proprius (principal sensory nucleus).
The neospinothalamic tract is the major ascending pathway involved in conveying pain signals to the higher centers.It arises from the nucleus proprius (principal sensory nucleus). The axons of the principal sensory nucleus, which mediate nociceptive signals, cross to the contralateral side in the anterior (ventral) white commissure of the spinal cord and form the neospinotha-lamic tract in the lateral funiculus. The neospinothalamic tract then ascends through the medulla, pons, and the midbrain and projects upon neurons located in the ventral posterolateral nucleus and posterior nuclei of the thala-mus. Axons of the thalamic neurons project to the primary sensory cortex (see Fig. 9-10).
Injury to the neospinothalamic tract in the brainstem or spinal cord results in loss of pain and thermal sensation on the contralateral side below the level of the lesion. Knowledge of the anatomical and functional properties of the neospinothalamic tract has been applied by neurosur-geons to eliminate intractable pain by surgically interrupting the spinothalamic tract (cordotomy) usually at the level of the spinal cord.
Anatomical Pathways Mediating Pain Sensation From the Head and Face. The main circuits mediating pain sensation from the head and face are the trigeminal thalamic pathways.Descending Pathways Modulating Pain Sensory Mechanisms. Pain sensation is modulated by the following descending pathways.
Pathway from the Periaqueductal Gray. The neurons located in the periaqueductal gray matter (PAG) of the midbrain project to the nucleus raphe magnus, which is located in the medulla. Electrical stimulation of PAG in human subjects and experimental animals is known to suppress the activity of nociceptive mechanisms (i.e., analgesia is produced). Therefore, stimulation of the PAG is believed to excite neurons in the raphe magnus, which, in turn, modulate pain sensation (see the following sections).
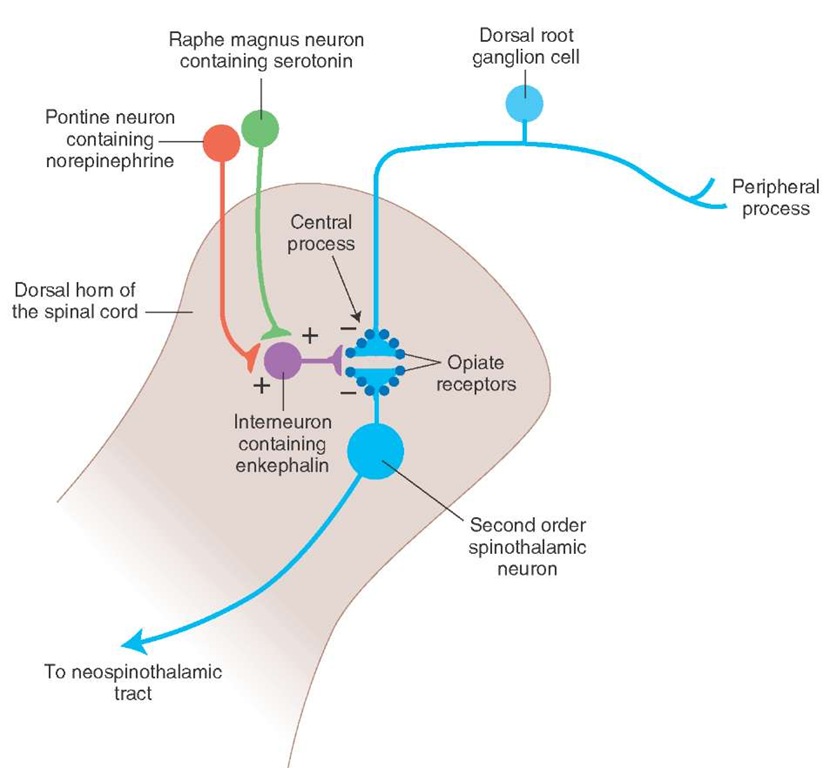
Pathway from the Nucleus Raphe Magnus. The anatomical course of this pathway is shown in Figure 15-3. The nucleus raphe magnus lies in the midline medulla, and many neurons located in this nucleus are serotonergic. The axons of these neurons descend to all levels of the spinal cord and synapse on enkephalin (an endogenous opioid peptide containing interneurons located in the dorsal horn. The enkephalinergic interneurons form axo-axonal type synapses on the primary afferent terminals of pain fibers and axo-dendritic type synapses on the second-order dorsal horn neurons mediating the pain sensation. Stimulation of the descending serotonergic projections from the raphe magus excites enkephalinergic interneurons in the spinal cord. Enkephalins released from these interneurons inhibit the release of transmitter from the central processes of nociceptive dorsal root ganglion neurons. Stimulation of the enkephalinergic interneurons also inhibits the second-order spinothalamic dorsal horn neurons via a postsynaptic mechanism (see "Neuro-transmitters Involved in Pain Pathways"). Thus, stimulation of the raphe magnus neurons produces analgesia by inhibiting the dorsal horn neurons from which the spinothalamic tract arises.
NoradrenergicPathway. Axons of noradrenergic locus ceru-leus neurons located in the upper pons descend through the medulla to the dorsal horn of the spinal cord. Stimulation of this descending noradrenergic pathway inhibits dorsal horn neurons that relay pain sensation by activating enkephalinergic interneurons via the mechanism described in the previous section ("Pathway from the Nucleus Raphe Magnus").
Neurotransmitters Involved in Pain Pathways. Our current knowledge regarding various transmitters released at different sites in the neuronal circuits mediating pain sensation can be summarized as follows (Fig. 15-4). The neurotransmit-ters released in the dorsal horn of the spinal cord, at the terminals of central processes of first-order noci-ceptive neurons (located in dorsal root ganglia), are believed to be glutamate and substance P. These neurotransmitters excite second-order spinothalamic dorsal horn neurons. The axons of these second-order neurons cross to the con-tralateral side and form the ascending neospinothalamic tract. Opiate receptors are present on the terminals of the central processes of the first-order nociceptive dorsal root ganglion neurons (presynaptic opiate receptors) and on the dendrites of second-order spinothalamic neurons (postsynaptic opiate receptors).
FIGURE 15-3 Descending pathways modulating pain sensory mechanisms. The neurons located in the periaqueductal gray matter of the mid-brain project to the serotonergic neurons in the nucleus raphe magnus that is located in the midline of the medulla. Locus ceruleus noradrener-gic neurons are located in the upper pons. The axons of serotonergic raphe magnus neurons and noradrenergic locus ceruleus neurons descend to all levels of the spinal cord and synapse on enkephalin-containing interneurons located in the dorsal horn.
The enkephalinergic interneurons located in the dorsal horn make axo-axonal and axo-dendritic synapses at the terminals of the central processes of the first-order nociceptive dorsal root ganglion neurons and dendrites of second-order spinothalamic neurons, respectively. The enkephalinergic interneurons are activated by the projections from the medullary serotonin-containing nucleus raphe magnus and pontine locus ceruleus noradrenergic neurons. Enkephalin released from terminals of enkepha-linergic dorsal horn interneurons acts on the opiate receptors located on the central processes of the nociceptive neurons located in the dorsal root ganglia, reduces Ca2+ (calcium) entry into the terminal, and decreases the release of neurotransmitters (glutamate and/or substance P). Enkephalin released from terminals of these dorsal horn interneurons also activates postsynaptic opiate receptors on the dendrites of the second-order spinothalamic neurons, hyperpolarizes them by increasing K+ conductance, and inhibits them. These actions of enkephalin attenuate the effects of nociceptive stimuli. Thus, stimulation of descending serotonergic and noradrenergic projections to the dorsal horn results in the stimulation of enkephalinergic interneurons in the dorsal horn, and enkephalin released from them inhibits second-order spinothalamic neurons by presynaptic and postsynaptic mechanisms. Some Pain Syndromes.
FIGURE 15-4 Neurotransmitters involved in pain pathways. Substance P and glutamate are released in the dorsal horn of the spinal cord in response to peripheral painful stimulus. Descending projections from the nucleus raphe magnus and locus ceruleus activate enkephalinergic interneurons in the dorsal horn. Enkephalin released from these interneurons inhibits the release of substance P and glutamate.
Hyperalgesia. Enhancement of the sensation of pain is called hyperalgesia, which results from tissue damage and the release of many endogenous chemicals. These chemicals may activate nociceptors themselves or may sensitize the nociceptors (e.g., lower their threshold). One of the endogenous substances known to sensitize nociceptors, causing hyperalgesia, is prostaglandin E2 (a cyclo-oxygenase metabolite of arachidonic acid). Aspirin and other nons-teroidal anti-inflammatory analgesics inhibit the enzyme cyclo-oxygenase and prevent the synthesis of prostagland-ins. This effect may be responsible for their analgesic effect. Other endogenous chemicals that produce hyperalgesia are histamine, substance P, serotonin, and bradykinin.
Phantom Limb Pain. When a limb is amputated in some people, they experience the sensation of pain emerging from the missing limb. This is called phantom limb pain. It is believed that overactivity of the dorsal horn neurons on the side of the amputated limb may create a false feeling in the person that the pain is emanating from the amputated limb.
Causalgia (Sympathetic Dystrophy Syndrome). The burning sensation in causalgia is caused by increased sympathetic efferent activity after a peripheral nerve injury.
Neuralgia. Neuralgia is characterized by severe persistent pain in the distribution of a cranial or spinal nerve. For example, in trigeminal neuralgia, the pain (which comes in episodes and feels like stabbing) is precipitated by activities such as eating or brushing one’s teeth and is limited to the sensory distribution of the trigeminal nerve located in the face. Surgical interruption of the trigeminal nerve or medullary spinal tract (trigeminal tractotomy) is sometimes done to reduce or eliminate trigeminal neuralgia. Oral administration of anticonvulsants (e.g., carbamaze-pine or phenytoin) can alleviate paroxysmal pain by limiting aberrant transmission of nerve impulses. Electro-coagulation of the trigeminal ganglion and injection of alcohol in the trigeminal nerve have also been used to alleviate trigeminal neuralgia.
Thalamic Pain Syndrome. Lesions in the posterior thalamus may cause chronic pain in some patients.The etiology of thalamic pain syndrome and its nature are poorly understood.
Referred Pain. Sometimes, pain arising from deep visceral structures is felt at sites on the surface of the body. For example, pain stimuli arising due to myocardial ischemia are felt radiating to the sternum, arms, and wrists. This is called referred pain. One explanation for this phenomenon is that the sensory pain fibers innervating the heart follow the sympathetic innervation of this organ back to the spinal cord, and their cell bodies are located in thoracic dorsal root ganglia at T1—T5. The neuronal cell bodies supplying the dermatomes of the upper thorax and upper limbs are also located in the same dorsal root ganglia (T1-T5) and synapse on the same second-order neurons in the spinal cord segments (T1—T5) where cardiac sensory pain fibers synapse. Because of this anatomical arrangement, the CNS structures involved in the perception of the pain sensations cannot clearly differentiate between the pain signals arising from the visceral organs and the dermatomes of the upper thorax and upper limbs, so the pain sensation is incorrectly ascribed to the aforementioned dermatomes instead of a deeper visceral structure. This is because the inputs from the cutaneous nociceptors are more abundant than those from the visceral nociceptors.
Headaches. The International Headache Society has listed about 150 types of headaches. They are broadly classified as primary and secondary headaches and cranial neuralgias. The primary headaches (i.e., headaches not attributed to any underlying condition) include tension headaches, migraines, and cluster headaches. The secondary headaches are caused by numerous factors, such as head or neck trauma, cranial blood vessel disorders, or infections. Cranial neuralgias are the result of nerve pain in the head region.
The most common symptom of headache is pain. The frequency, intensity, and duration of pain may vary. The patients often describe the pain as pounding, throbbing, steady, or intense. The pain may occur unilaterally or bilaterally. It may be restricted to focal areas or may be generalized. The pain may be accompanied by other symptoms, such as nausea, vomiting, temporary visual disturbances, irritability, restlessness, excessive sweating, and fatigue. Some of the common headaches are briefly discussed below.
Tension headache: This is the most common type of headache. Characterized by a dull, pressure-like pain in the head, neck, and scalp, it typically develops early in the day and is more common in women.
Migraine: This is the second most common type of headache. It is more common in women and individuals between 15 and 55 years of age who have a parent or sibling suffering from this condition. Migraine attacks often include the following features: (1) prodromal phase (characterized by a change in mood that begins hours or days before the headache and includes symptoms such as depression, sleepiness, talkativeness, and restlessness), (2) aura characterized by visual abnormalities, such as shimmering spots or stars, zigzag lines that float across the patient’s field of vision, blind spots (scotomas), flashes of light, blurred vision, loss of vision in one eye, numbness or tingling in parts of the body, dizziness, and speech problems, and (3) headache phase. The headache itself may typically start on one side of the forehead but may affect both sides of the head. The pain starts gradually and may last from 4 hours to 3 days in adults. The headache is often accompanied by loss of appetite, nausea, vomiting, sensitivity to light and sound, blurred vision, and tenderness of the scalp or neck. Although vasodilation of meningeal arteries is considered to be one of the causes of migraine headaches, a neurogenic basis of the condition is also recognized. The following drugs are usually prescribed to relieve migraine headaches: 5-HT (serotonin receptor) agonists (triptans) and ergot alkaloids and ergot derivatives (which cause constriction of cranial blood vessels), analgesics (e.g., a combination of aspirin, butalbital, and caffeine), and nonsteroidal anti-inflammatory drugs (e.g., ibuprofen and naproxen sodium).
Cluster headache: This condition is characterized by a cyclic pattern (clusters) of headache. The clusters may last for weeks or months and then stop completely during the periods of remission, which may last for months or years. The pattern of clusters varies among the patients. Cluster headache is one of the most painful types of headache. The symptoms include intense, sharp, burning pain, generally located in or around the eye, but may radiate to other areas of the face, head, neck, and shoulders. The pain may be one-sided and accompanied by restlessness, excessive lacrimation, redness in the eye, swelling around the eye, pupillary constriction, drooping eyelid, and nasal congestion. During the attack, the patients are often restless and pace or sit and rock back and forth to soothe the attack. Lying down during an attack exacerbates the pain. Cluster headaches are relatively rare and non-life-threatening. Treatments can help make the attacks shorter and less severe. In addition, preventive medications can help reduce the number of headaches. Inhalation of 100% oxygen commonly provides relief. Drugs that may relieve cluster headache include ergotamine; sumatriptan (Imitrex); corticosteroids, such as prednisone; and methysergide.
Sinus headache: This condition often accompanies inflammation of the sinuses (sinusitis) caused by colds and allergies. The symptoms include pain over the cheeks, forehead, and, sometimes, eyes.
Post-lumbar puncture headache: This type of headache may appear within 1 week after a lumbar puncture (spinal tap) to remove some cerebrospinal fluid (CSF). Decrease in CSF pressure is believed to precipitate this headache. The headache usually disappears within 2 weeks. Sitting in an upright position, standing, and straining during cough exacerbate the pain, whereas lying in a recumbent position relieves it.
Temperature
The signals from cold and hot stimulus are carried by small myelinated AS fibers and unmyelinated C fibers. These fibers enter the tract of Lissauer, branch, and then ascend or descend one to three segments and terminate in the dorsal horn. The anatomic pathways that mediate temperature sensations are identical to those that mediate pain sensation. The pathways mediating temperature sensation also mediate crude touch from naked nerve endings.
Clinical Case
History
Annie is a 56-year-old woman who has suffered from diabetes for over 30 years. For the past fewyears,she has noticed that she frequently experienced numbness, tingling, and pain in her feet. However, more recently, she noticed that she was suffering from ulcers on her feet, and was frequently tripping. While carrying a large box down a steep staircase, she missed a step and fell down several steps. She called for help and was taken to the local emergency room (ER).
Examination
At the ER,the examining physician noted that Annie had several large bruises on her head, back, and extremities but was alert and oriented.She had several ulcers on her feet. Although she could feel a hand placed on her feet, she was unable to tell which way her toes were moved if her eyes were closed. She was also unable to feel a vibrating tuning fork placed on her ankles but was able to feel the vibrations slightly when the tuning fork was placed on her knees. Her gait was a bit unsteady but improved when she looked down as she walked. When the doctor asked Annie to stand with her feet together and her eyes closed, she became very unsteady. Although there was a trace of knee-jerk reflexes bilaterally, the ankle-jerk reflexes were absent.
Explanation
Annie’s neurologic problem is a classic example of a peripheral neuropathy (dysfunction of the peripheral nerves without involvement of the central nervous system).This condition is often described as"stocking glove"in nature because of the anatomic pattern it follows in which the affected parts include the hands and feet. Diabetes is a common cause of peripheral neuropathy.The exact mechanism is unknown, but the disease preferentially affects the larger myelinated fibers, prior to the smaller motor fibers, and A5 and unmyelinated C fibers, which govern primarily pain and temperature. Although there is a sensation of numbness, examination revealed that the primary deficits lie with position and vibratory senses. Because there is some degree of numbness, it is not uncommon for diabetics to suffer from foot ulcers, since it is difficult to recognize poorly fitting shoes. The gait described is called sensory ataxia and is an unsteady gait resulting from a deficiency in identifying the location of feet on the floor. When Annie walked down the stairs carrying a large box, she was most likely mechanically unable to look down at her feet and thus could not feel their location on the stairs, causing her to trip. The ankle-jerk reflexes are lost due to loss of the sensory component of the deep tendon reflex. In Romberg’s test, the patient is asked to stand with feet together and eyes closed; it is a test of position sense. If cerebellar and motor function are normal (as we presume that they are in this case), the reason for unsteadiness is the loss of position sense. Because the same fibers carry position and vibratory sense, the latter is lost as well. In more advanced cases of diabetic neuropathy, motor function and pain and temperature sensation may be lost as well.
SUMMARY TABLE
Selected Pain Syndromes
|
Pain Syndrome |
Salient Features |
|
Hyperalgesia |
Enhancement of pain sensation; results from tissue damage and release of many endogenous chemicals; chemicals may activate or sensitize nociceptors; one of endogenous substances known to sensitize nociceptors is prostaglandin E2 (a cyclo-oxygenase metabolite of arachidonic acid); aspirin and other nonsteroidal anti-inflammatory analgesics inhibit enzyme cyclo-oxygenase and prevent synthesis of prostaglandins (which may be responsible for their analgesic effect); other endogenous chemicals that produce hyperalgesia are histamine, substance P, serotonin,and bradykinin |
|
Phantom limb pain |
Sensation of pain emerging from amputated limb; overactivity of dorsal horn neurons on side of amputated limb may create a false feeling that pain is emanating from amputated limb |
|
Causalgia (sympathetic dystrophy syndrome) |
Burning sensation caused by increased sympathetic efferent activity after a peripheral nerve injury |
|
Trigeminal neuralgia |
Characterized by severe persistent pain in distribution of trigeminal nerve; pain comes in episodes and feels like stabbing; precipitated by activities such as eating or brushing one’s teeth and is limited to sensory distribution of trigeminal nerve located in face; surgical interruption of trigeminal nerve or medullary spinal tract (trigeminal tractotomy) is sometimes done to reduce or eliminate trigeminal neuralgia; oral administration of anticonvulsants (e.g.,carbamazepine or phenytoin) can alleviate paroxysmal pain by limiting aberrant transmission of nerve impulses; electrocoagulation of trigeminal ganglion and injection of alcohol in trigeminal nerve have also been used to alleviate trigeminal neuralgia |
|
Thalamic pain syndrome |
Lesions in posterior thalamus may cause chronic pain in some patients; etiology of this syndrome and its nature are poorly understood |
|
Referred pain |
Sometimes pain arising from deep visceral structures is felt at sites on surface of body, e.g., myocardial ischemic pain is felt radiating to sternum, arms, and wrists; one explanation for this phenomenon is that sensory neurons innervating heart are located in same dorsal root ganglia where neurons innervating dermatomes of the upper thorax and upper limbs are located (T1-T5); because of this anatomical arrangement, cardiac pain sensation is incorrectly ascribed to dermatomes of upper thorax and upper limbs |
|
Primary headaches |
Not attributed to any underlying condition |
|
Tension headache |
Typically characterized by a dull, pressure-like pain in head, neck,and scalp; develops early in day and is more common in women |
|
Migraine headache |
More common in women and individuals who have a parent or sibling suffering from this condition; symptoms include: (1) prodrome (characterized by a change in mood that begins hours or days before headache and includes symptoms such as depression, sleepiness, talkativeness, and restlessness), (2) aura characterized by visual abnormalities, such as shimmering spots or stars, zigzag lines that float across patient’s field of vision, blind spots, flashes of light, blurred vision, loss of vision in one eye, numbness or tingling in parts of body, dizziness, and speech problems, and (3) headache phase, which may affect one or both sides of head; pain starts gradually and may last from 4 hours to 3 days in adults; often accompanied by loss of appetite, nausea, vomiting, sensitivity to light and sound, blurred vision, and tenderness of scalp or neck; causes include neurogenic factors and vasodilation of meningeal arteries; drugs usually prescribed to relieve migraine headaches: serotonin receptor agonists (triptans) and ergot alkaloids and ergot derivatives (which cause constriction of cranial blood vessels), analgesics (e.g., a combination of aspirin, butalbital, and caffeine), and nonsteroidal anti-inflammatory drugs (e.g., ibuprofen and naproxen) |
|
Pain Syndrome |
Salient Features |
|
Cluster headache |
Characterized by a cyclic pattern (clusters) of headache; clusters may last for weeks or months and then stop completely during periods of remission, which may last for months or years; symptoms include intense, sharp, burning pain, generally located in or around eye, but may radiate to other areas of face, head, neck,and shoulders; pain may be one-sided and accompanied by restlessness, excessive lacrimation, redness in eye, swelling around eye, pupillary constriction, drooping eyelid, and nasal congestion; lying down during an attack exacerbates pain; drugs that may relieve cluster headache include ergotamine, sumatriptan (Imitrex), corticosteroids, (prednisone), and methysergide |
|
Sinus headache |
Accompanies inflammation of sinuses (sinusitis) caused by colds and allergies; symptoms include pain over cheeks, forehead, and, sometimes, eyes |
|
Post-lumbar puncture headache |
Appears within 1 week after a lumbar puncture to remove some CSF; headache is precipitated by decrease in CSF pressure; headache disappears within 2 weeks;sitting in an upright position, standing,and straining during cough exacerbate pain, whereas lying in a recumbent position relieves it |
|
Secondary headaches |
Caused by numerous factors, such as head or neck trauma, cranial blood vessel disorders, or infections |
|
Cranial neuralgia |
Results from nerve pain in head region |
T = thoracic segment of spinal cord;CSF = cerebrospinal fluid.