Receptors
Receptors are proteins located on neuronal membranes with which neurotransmitters bind; the result is the opening or closing of specific ion channels. There are two families of receptors: (1) ionotropic or ligand-gated receptors, and (2) metabotropic or G-protein-coupled receptors.
Ionotropic Receptors
Ionotropic receptors usually consist of multimeric proteins (different proteins, usually five). Each subunit spans the plasma membrane and contributes to the formation of the pore of the ion channel. They are called ligand-gated receptors because they combine transmitter-binding and channel functions into one molecular entity. When the neurotransmitter binds with the receptor site, an ion channel opens and a response is elicited. These types of receptors generally mediate rapid and short-duration responses. Examples of ionotropic receptors for different neurotransmitters are shown in Table 8-3. Some of these receptors are discussed in the following sections.
TABLE 8-3 Ionotropic and Metabotropic Receptors for Different Neurotransmitters
AMPA = alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate; GABA = gamma aminobutyric acid; NMDA = N-methyl-D-aspartic acid.
Nicotinic Acetylcholine Receptor
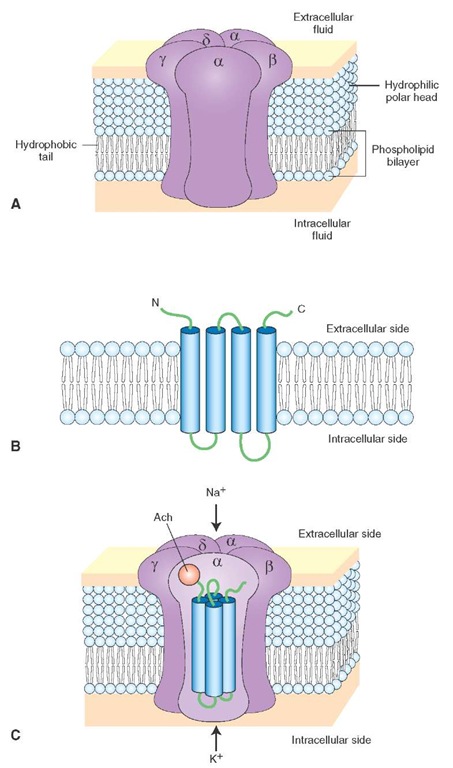
These receptors are located at the neuromuscular junction (NMJ) as well as at central neurons. Nicotinic ace-tylcholine receptor (nAChR) at the NMJ consists of five protein subunits: two alpha, one beta, one gamma, and one delta subunit (Fig. 8-17A). Each subunit spans the membrane four times (Fig. 8-17B). Twenty trans-membrane domains from five subunits surround a central pore of the channel (Fig. 8-17C); the transmembrane domains of only one a-subunit are shown in the figure. Ach binds to the a-subunits and opens the channel to allow influx of Na+ and efflux of K+ (agonists: Ach and nicotine; antagonist: d-tubocurarine, which is one of the muscle relaxants used in surgical procedures).
Clinical Disorders Associated With the Neuromuscular Junction. There are two major autoimmune disorders associated with the NMJ. In Lambert-Eaton syndrome, an antibody develops against the Ca2+ channels on the presynaptic terminal at the NMJ.In myasthenia gravis, antibodies develop against the nAChR at the NMJ.
N-Methyl-D-Aspartic Acid Receptor
N-methyl-D-aspartic acid (NMDA) receptor, an iono-tropic glutamate receptor, is distributed widely in the CNS. Current evidence suggests that the NMDA receptor consists of five subunits (NMDAR1, NMDAR2A, NMDAR2B, NMDAR2C, and NMDAR2D). Each NMDA receptor subunit consists of four transmembrane domains (TM1-TM4). The TM2 domain forms a kink and does not fully traverse the membrane (Fig. 8-18). The TM2 domain of each subunit lines the pore of the NMDA receptor channel. The activity of this receptor can be altered through the following binding sites (Fig. 8-18):
1. Transmitter binding site where L-glutamate and related agonists act and promote opening of the channel through which Na+ and Ca2+ ions enter and K+ ions leave the cell.
2. A region of the transmitter binding site where NMDA receptor antagonists bind.
3. The strychnine-insensitive glycine modulatory site, which must be occupied by glycine in order for L-gluta-mate to be effective at this receptor. Glycine increases the frequency of the opening of this receptor channel. At the glycine binding site of the NMDA receptor, strychnine does not act as an antagonist; therefore, it is called the strychnine-insensitive glycine site. It should be noted that, in the synapses where glycine acts as a neurotransmitter, strychnine acts as an antagonist, and these sites are called strychnine-sensitive glycine sites (see section, "Glycine Receptor").
4. Voltage-dependent magnesium binding site where Mg2+ binds at normal resting potentials or when the cell is hyperpolarized and blocks the NMDA receptor channel. Therefore, NMDA receptor cannot be activated at normal resting potentials or when the cell is hyperpolarized. When the cell is depolarized, Mg2+ is dislodged from its binding site in the channel, and Na+ and Ca2+ enter, while K+ leaves the cell through the same channel.
5. The phencyclidine binding site is located within the channel where phencyclidine (also known as "PCP" and "angel dust") binds and elicits the side-effects associated with its use.
Kainate Receptor
The binding of kainic acid to kainate receptor, an iono-tropic glutamate receptor, results in the opening of an ion channel, permitting influx of Na+ (but not Ca2+) and efflux of K+ (potassium) through the same channel, and the neuron is depolarized.
FIGURE 8-17 Nicotinic acetylcholine receptor (nAChR). (A) nAChR at the neuromuscular junction consists of five protein subunits (alpha, beta, gamma, and delta). (B) Each subunit spans the membrane four times. N and C represent amino (NH2) and carboxyl (COOH) terminals, respectively. (C) The transmembrane domains from five subunits surround a central pore of the channel. Acetylcholine (Ach) binds to the a-subunit and opens the channel to allow influx of Na+ (sodium) and efflux of K+ (potassium).
FIGURE 8-18 Components of an N-methyl-D-aspartic acid (NMDA) receptor. PCP = phencyclidine; Ca2+ = calcium; Na+ = sodium; K+ = potassium; Mg2+ = magnesium.
FIGURE 8-19 Components of a gamma aminobutyric acid type A (GABAA) receptor. Cl- = chloride.
AMPA/Quisqualate Receptor
AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate) and quisqualic acid are agonists for AMPA/ quisqualate receptor, an ionotropic glutamate receptor. Binding of these agonists to this receptor results in the opening of an ion channel, permitting influx of Na+ (but not Ca2+) and efflux of K+, and the neuron is depolarized.
GABAa Receptors
GABAA receptors, ionotropic receptors, are distributed throughout the CNS. They consist of a combination of five subunits: two alpha, two beta, and one gamma (Fig. 8-19). Each subunit has four transmembrane domains (TM1-TM4). Present on these receptors are major binding sites: (1) for agonists (e.g., GABA), (2) for antagonists (e.g., bicuculline), (3) for barbiturates (e.g., phenobarbi-tal), and (4) for benzodiazepines (e.g., diazepam [Valium]). Activation of the GABA receptor site by GABA agonists results in the opening of the chloride channel; negatively charged chloride ions (Cl-) enter the neuron and hyperpo-larize and inhibit it. GABA receptor antagonists bind to their receptor site, and the conformation of the receptor changes so that subsequent application of the GABA receptor fails to elicit a response. Barbiturates bind to another site and prolong the opening of chloride channel. Antianxiety (anxiolytic) drugs (e.g., diazepam) that bind to the benzodiazepine site enhance the electrophysiologi-cal effects of GABA on these neurons.
Glycine Receptor
Glycine has been implicated as a neurotransmitter in the spinal cord, lower brainstem, and retina. Activation of glycine receptors results in an influx of chloride ions into the neuron, which is then hyperpolarized and inhibited. Strychnine blocks the glycine receptors at the synapses where glycine acts as a neurotransmitter. As stated earlier, the glycine sites on the NMDA receptors are strychnine-resistant.
Serotonin Receptors
At least seven subtypes of serotonin receptors have been identified. Only 5-HT3 receptors are ionotropic receptors. All other subtypes are metabotropic receptors (see Table 8-3). 5-HT3 receptors are located in central and peripheral neurons. Their function remains to be established.
Metabotropic Receptors
These receptors do not have the ion channel as part of the receptor. The flow of ions through the channels associated with these receptors depends on one or more metabolic steps. Therefore, these receptors are called metabotropic receptors. The opening or closing of the ion channels associated with these receptors involves activation of intermediate molecules called G-proteins. For this reason, metabotropic receptors are also called G-protein-coupled receptors.
Metabotropic receptors consist of a single protein (monomeric) molecule that has membrane-spanning domains (I-VII) (Fig. 8-20A). Portions of domains II, III, VI, and VII make up the extracellular domain where a neurotransmitter binding site is located. G-proteins bind to the intracellular loop between domain V and VI and to portions of the C terminus. Heterotrimeric G-proteins consist of three subunits: alpha, beta, and gamma (Fig. 8-20B). The a-subunit binds guanine nucleotides, such as GTP and guanosine-5′-diphosphate (GDP). When GDP is bound to the a-subunit, the three subunits are bound together and form an inactive trimer.
The steps involved in the binding of the neurotransmitter to the metabotropic receptor and the events that follow are shown in Figure 8-20C. The neurotransmitter binding to the metabotropic receptor results in the replacement of GDP by GTP on the a-subunit. The activated GTP-a-subunit dissociates from the P-y-subunit complex. Either one of these complexes can bind to effector molecules (e.g., enzymes), stimulate them (e.g., adenylate cyclase), and generate second messengers (e.g., cyclic adenosine monophosphate [cAMP]). The second messengers (e.g., cAMP) stimulate enzymes (e.g., protein kinase-A), which then phosphorylate appropriate ion channels.
FIGURE 8-20 Metabotropic receptors. (A) A single protein (monomeric) molecule with seven membrane-spanning domains (I—VII). A neurotransmitter-binding site is located in the extracellular domain (made up of portions of domains II, III, VI, and VII). G-proteins bind to the intracellular loop between domains V and VI and to portions of the C terminus. N and C denote NH2 and COOH terminals, respectively. (B) Heterotrimeric G-proteins consist of three subunits (alpha, beta, and gamma). When guanosine-5′-diphosphate (GDP) is bound to the a-subunit, the a-subunit binds to P- and y-subunits, and an inactive trimer is formed. (C) The steps of a neurotransmitter binding to the metabotropic receptor and the events that follow. GTP = guanosine triphosphate.
Usually phosphorylation of ion channels by protein kinases results in opening of the channel; ions flow across the neuronal membrane, the neurons are depolarized, and the amplitude of neurotransmitter-induced excitatory postsynaptic potentials (EPSPs) is increased. Hence, the neuron is made more excitable. The onset and duration of responses mediated by the metabotropic receptors is longer than those mediated by ionotropic receptors. Dephospho-rylation of ion channels by protein phosphatases results in the closing of the channel.
Examples of metabotropic receptors for different neurotransmitters are shown in Table 8-3.
Some of them are discussed in the following sections.