Introduction
The prion hypothesis states that the partially protease-resistant and detergent-insoluble prion protein (PrPSc) is identical with the infectious agent, and lacks any detectable nucleic acids. Since the latter discovery, transgenic mice have contributed many important insights to the field of prion biology. The prion protein (PrPC) is encoded by the Prnp gene, and disruption of Prnp leads to resistance to infection by prions. Ectopic expression of PrPC in PrPC-knock-out mice proved a useful tool for the identification of host cells competent for prion replication. Finally, the availability of PrPC-knockout mice, and transgenic mice overexpressing PrPC, allowed selective reconstitution experiments aimed at expressing PrPC in neurografts or in specific populations of hemato- and lymphopoietic cells. The latter studies helped elucidate some of the mechanisms of prion spread and disease pathogenesis.
PrP-Knockout Mice and Their Phenotypes
If the protein only hypothesis is correct, PrPC functions as a substrate for the PrPSc-mediated conversion of PrPC into new PrPSc molecules. As a consequence of this hypothesis, an organism lacking PrPC should be resistant to scrapie and unable to propagate the infectious agent. The mice generated by Bueler et al. (1) carry a targeted disruption of the Prnp gene. This was achieved by homologous recombination in embryonic stem cells. In the disrupted Prnp allele, 184 codons of the Prnp coding region (which consists of 254 codons) were replaced by a drug-resistance gene as selectable marker. A second line of PrPC knockout mice was generated by Manson et al. (2) by inserting a select able marker into the PrPC open reading frame, which leads to a disruption of the coding region of Prnp. Sakaguchi et al. created a third PrPC-knockout line, in which the whole PrPC open reading frame and about 250 bp of the 5′ intron and 452 bp of 3′ untranslated sequences were replaced with a drug-resistance gene (3). Although both the Bueler and Sakaguchi mice were on a mixed genetic (129/Sv x C57BL) background, the mice generated by Manson were bred on a pure 129/Ola background. According to the terminology that has become customary in the literature, and to which we abide in this manuscript, the Bueler mice have been designated Prnp0/0 while the Manson and the Sakaguchi mice are termed Prnp-/.
The phenotype of the Prnp-knockout mice was of great interest, because it was proposed that PrPC, which is a ubiquitously expressed neuronal protein, may have a housekeeping function (4).Yet the homozygous PrPC-knockout mice generated by Bueler et al. and Manson et al. were viable and showed no overt phenotypic abnormalities, suggesting that PrPC does not play a crucial role in development or function of the nervous system (1,2). The Prnp0/0 mice show no behavioral impairment and perform like the wild type controls in spatial learning tests (5). Detailed analysis revealed electrophysiological defects, such as weakened y-aminobutyric acid type A receptor-mediated fast inhibition and impaired long-term potentiation in the hippocampus for the Zurich and Edinburgh PrPC-knockout mice compared to their corresponding wild-type counterparts, indicating that PrPC may play a role in synaptic plasticity (6). Tobler et al. (7) reported altered sleep patterns and rhythms of circadian activity in the Bueler and Manson mice.
The PrPC-null mice derived by Sakaguchi et al. developed severe progressive ataxia starting from 70 weeks of age (3). Analysis of the brains of affected animals revealed extensive loss of cerebellar Purkinje cells (8). Because no such phenotype was observed in the other two lines of PrPC-knockout mice, it seems likely that this phenotype is not the result of the lack of PrPC, but rather results from deletion of flanking sequences. A Purkinje cell-specific enhancer was proposed (9) to be contained within the second intron of Prnp. The report (10) that expression of a Prnp transgene can rescue this phenotype argues against the hypothesis that the phenotype was caused by deletion of a regulatory element, rather than of the Prnp reading frame (10). Recently, evidence has been forthcoming that the phenotype observed in the Sakaguchi mice is caused by upregulation of a second Prnp-like gene located 16 kb downstream for the Prnp gene (11). The exact mechanism of this process is still under discussion (12).
PrP-Null Mice Are Resistant to Scrapie
One of the milestones in scrapie research was the inoculation of PrPC null mice with mouse adapted scrapie strains. All three PrPC null mouse lines were resistant to scrapie. The Prnp0/0 mice generated by Bueler et al., inoculated with the RML isolate of mouse-adapted prions, remained healthy for their whole life-span, and did not show any signs of scrapie-typical neuropathology (13). This observation was confirmed using different PrPC-null mice with different mouse-adapted scrapie inocula (3,14). Mice hemizygous for the disrupted Prnp gene (Prnp0/+) showed partial resistance to scrapie infection, as manifested by prolonged incubation times of ~290 d as compared to ~160 d in the case of Prnp+/+ mice. There is a strict correlation between the levels of PrPC in the host and incubation times until terminal disease; the severity of the disease, in terms of neuropathological changes in the brain and levels of prion infectivity, were not dependent on the PrPC level (14,15). All of the experiments show that the amount of PrPC present in the brain seems to be the rate-limiting step in the development of the disease. Therefore, therapeutic efforts aimed to reduce the amount of PrPC may be effective.
Structural Implications of the Infectivity of PrP
Limited proteolysis of PrPSc cleaves off the N-terminus and a fragment termed PrP27-30 remains. This portion of PrPSc is still infectious, meaning that the last 60 amino-proximal residues of PrPSc are not required for infectivity (16,17). PrPC lacking residues 23-88 can be converted into protease-resistant PrPC in scrapie-infected neuroblastoma cells (18). An important question arising from these experiments is whether N-terminally truncated PrPC molecules can support prion replication in mice. In order to address this question transgenic mice, expressing N-terminal deletions of the prion protein on a PrPC null background, were established. These mutant PrPC mice with amino-proxi-mal deletions of residues 32-80 and 32-93, corresponding to truncations of 49 and 63 residues, restore scrapie susceptibility, prion replication, and formation of truncated PrPScin PrPC deficient mice (19).
The data obtained from these experiments demonstrate that the octapeptide region encompassing residues 51-90 of murine PrPc is dispensable for scrapie pathogenesis. This is remarkable, because additional octapeptide repeats instead of the normal five segregate with affected individuals in families with inherited Creutzfeldt-Jakob disease (20), and because expression of a mutant PrPc with a pathological number of octarepeats induces a neurodegenerative disease in transgenic mice (21).
Mice Expressing Truncated PrPc Show Severe Ataxia
Nuclear magnetic resonance studies helped to reveal the three-dimensional structure of PrPC. Full-length, mature PrPC seems to have a highly flexible N- terminal tail that lacks ordered secondary structures extending from residue 23 to 121; the C-terminal part of PrPC consists of a stably folded globular domain (22,23). The highly flexible tail, part of which is protease-sensitive in PrPSc, comprises the most conserved region of PrPC across all species examined (24). Following these structural studies, the possibility that the flexible tail may play a role in the conformational transition of PrPC to PrPSc was proposed (22,25). To further analyze the importance of the flexible tail in regard to scrapie susceptibility, Shmerling et al. (19) generated amino-proximal deletions of residues 32-121 and 32-134, and expressed them as transgenes in PrPC-deficient mice. Mice overexpressing these transgenes developed severe ataxia and neuronal death limited to the granular layer of the cerebellum, as early as 1-3 mo of age. No pathological phenotype was observed in transgenic mice with shorter deletions encompassing residues 32-80, 32-93 and 32-106. Because of the selective degeneration of granule cells in the cerebellum, a nonspecific toxic effect elicited by the truncated PrPC can be ruled out. Another argument for a specific effect is the fact that neurons in the cortex, and elsewhere, express truncated PrPC at similar levels, but do not undergo cell death by apoptosis. One copy of a wild-type Prnp allele, introduced into these mice, completely abolishes the phenotype. Based on these results, a model was proposed in which truncated PrPC acts as dominant negative inhibitor of a functional homolog of PrPC, with both competing for the same putative PrPC ligand (19).
A different spontaneous neurologic phenotype was reported in mice carrying PrPC transgenes with internal deletions corresponding to either of the two carboxy-proximal a-helices. Two transgenic mouse lines generated on the Prnp0/0 background, expressing mutant PrPC with deletions of residues 23-88 and either residues 177-200 or 201-217, developed central nervous system (CNS) dysfunction and neuropathological changes characteristic of a neuronal storage disease (26). Because deletion of residues 23-88 alone did not lead to a spontaneous phenotype, it was concluded that ablation of either of the two C-terminal a-helices is sufficient to cause this novel CNS illness. Ultrastructural studies indicated extensive proliferation of the endoplasmic reticulum, and revealed accumulation of mutant PrPC within cytoplasmic inclusions in enlarged neurons.
A completely new light is shed on all of these studies by the discovery of a Prnp like gene, named Prnd that encodes for a protein named Doppel, (German for double) (Dpl), located 16 kb downstream of the murine Prnp gene (11). Elevated levels of Prnd RNA are present in certain strains of PrPC knockout mice that develop neurological symptoms. It was shown that mice with a truncated Prnp transgene, lacking the N-terminus, and therefore devoid of the conserved 106-126 amino acid region, develop granule cell degeneration. This phenotype can be rescued by introduction of single intact PrPC allele. The information from the PrPC knockout mice that overexpress Dpl and the data from the truncated Prnp transgenic mice, led to the hypothesis that PrPC interacts with a ligand to produce an essential signal. In PrPC knockout mice, a PrPC-like molecule, with a lower binding affinity, could substitute for PrPC. In the N-terminated transgenic mice, the truncated PrPC could bind the ligand with high affinity, without eliciting the survival signal. Dpl could act in a similar way, and produce its effects through a competition with PrPC for the PrPC ligand, thus blocking an important signal (11,12). Knockout mice for the Prnd gene should help to clarify whether this proposed model is accurate.
Neurografts in Prion Research
Prnp0/0 mice show normal development and behavior (1,2), which has led to the hypothesis that scrapie pathology is caused by PrPSc deposition, rather than by depletion of cellular PrPC (6). This was confirmed by cell culture experiments, which showed that a part of PrPC, corresponding to amino acids 106-126, acts as a neurotoxin. The peptide corresponding to residues 106-126 of PrPC has a high intrinsic ability to polymerize into amyloid-like fibrils (27). If the depletion of cellular PrPC is really the reason for scrapie pathology, lack of PrPC may result in embryonic or perinatal lethality, especially since PrPC is encoded by a unique gene, for which no related family members have been found. Until now, there is no stringent mouse model in which PrPC can be depleted in an acute fashion. In this case, the depletion of PrPC may be much more deleterious than its lack throughout development, because the organism may then not have the time to enable compensatory mechanisms.
The neurografting technique offers an attractive model to study the question of neurotoxicity of PrPSc. By grafting, one can expose brain tissue of Prnp0/0 mice to a continuous source of PrPSc. Mid-gestation neuroectoderm, overexpressing PrPC, was grafted into the brain of PrPC-deficient mice, using well-established protocols (28,29). Following intracerebral inoculation with scrapie prions, neuroectodermal grafts accumulated high levels of PrPSc and infectivity, and developed severe histopathological changes characteristic of scrapie. It was shown that, at later time-points, substantial amounts of graft-derived PrPSc migrated into the host brain, and, even in areas distant from the grafts, substantial amounts of infectivity were detected (9,30). Nonetheless, even 16 mo after transplantation and infection with prions, no pathological changes were detected in the PrPC-deficient tissue, not even in the immediate vicinity of the grafts or the PrPSc deposits. These results suggest that PrPSc is inherently nontoxic, and that PrPSc plaques found in spongiform encephalopathies may be an epiphenomenon, rather than a cause of neuronal damage (31). Maybe the PrPSc-containing plaques must be formed and localized intracellularly in order to be neurotoxic. If this is the case, plaques that are localized extracellularly may not be toxic. This would explain the absence of pathological changes outside the PrPC-con-taining grafts.
Because the host mice harboring a chronically scrapie-infected neural graft did not develop any signs of disease, they enabled us not only to study the effects of prions on the surrounding tissue, but were also an ideal model to assess changes occurring during the progression of scrapie disease in neuronal tissue. The possibility of studying late time-points, after infection with the scrapie agent, was useful in order to observe phenomena that cannot be seen in PrPC-containing mice, because these mice develop clinical symptoms, eventually leading to earlier death. With increasing incubation time, grafts underwent progressive astrogliosis and spongiosis, which were accompanied by loss of neuronal processes within the grafts and subsequent destruction of the neuropil (Fig. 1). The latest studied time-point was 435 d after inoculation; grafts showed an increase of cellular density probably caused by astroglial proliferation and a complete loss of neurons. Magnetic resonance imaging in vivo, using gadolinium as a contrast enhancing medium, revealed a progressive disruption of the blood-brain barrier in scrapie-infected grafts during the course of the disease (32). These findings confirmed several predictions about the pathogenesis of spongiform encephalopathies, primarily that scrapie leads to selective neuronal loss, and that astrocytes and perhaps other neuroectodermal cells, while being affected by the disease, can survive and maintain their phenotypic characteristics for long periods of time.
In other experimental models such as experimental hamster scrapie, disruption of the blood-brain barrier was also visible (33) yet no such observations were made in human spongiform encephalopathies. The localized blood-brain barrier disruption in chronically infected grafts may contribute to the spread of prions from grafts to the surrounding brain, as described previously (30). It may also account for the pattern of accumulation of protease-resistant PrP0 within the white matter, and in brain areas surrounding the grafts. The accumulation of PrPSc in nonaffected neuropil surrounding the graft could also be explained through vasogenic diffusion from the affected graft toward the host brain.
Mechanism of Prion Spread in the Central Nervous System
Transmission of prion diseases can be accomplished by injecting infected brain homogenate into suitable recipients. It has been shown that infection is possible by a number of different inoculation routes. Intracerebral inoculation is the most effective method for transmission of spongiform encephalopathies and may even facilitate circumvention of the species barrier. Other modes of transmission are oral uptake of the agent (34-36) intravenous and intraperito-neal injection (37) as well as conjunctival instillation (38), implantation of corneal grafts (39), and intraocular injection (40). Intraocular injection is an elegant way of studying the neural spread of the agent, because the retina is a part of the (CNS), and intraocular injection does not produce direct physical trauma to the brain, which may disrupt the blood-brain barrier and impair other aspects of brain physiology. The assumption that the spread of prions within the CNS occurs axonally rests on experimental data gathered from mainly mice intraocularly. Fraser (40) could show that the sequential development of spongiform changes follows the retinal pathway in a fashion that suggests transport along axons or in axons.
Fig. 1. Noninfected and scrapie-infected neural grafts in brains of Prnp0/0 mice. Upper row (A,B) Healthy control graft 230 d after mock inoculation. The graft is located in the third ventricle of the recipient mouse (A, see asterisks, hematoxylin and eosin), and shows no spongiform change, little gliosis (B, immunostain for glial fibrillary acidic protein [GFAP]) (C,D) Scrapie-infected graft 235 d after inoculation with increased cellularity (C), brisk gliosis (D). Bottom row: High magnification of a similar graft shows characteristic pathological changes in a chronically infected graft. (E) Appearance of large vacuoles and ballooned neurons (arrow). In the GFAP immunostain (F), astrocytes appear wrapped around densely packed neurons.
It has been repeatedly shown that expression of PrPC is required for prion replication (13,41) and for neurodegenerative changes to occur (30). To investigate whether spread of prions within the CNS is dependent on PrPC expression in the visual pathway, PrPC-producing neural grafts were used as sensitive indicators of the presence of prion infectivity in the brain of an otherwise PrPC-deficient host.
Following inoculation with prions into the eye of grafted Prnp0/0 mice, none of the grafts showed signs of spongiosis, gliosis, synaptic loss, or PrPSc deposition. In one instance, the graft of an intraocularly inoculated mouse was assayed and found to be devoid of infectivity. Therefore, it was concluded that infectivity administered to the eye of PrPC-deficient hosts cannot induce scrapie in a PrPC-expressing brain graft (42).
One problem encountered while conducting work with PrPC-containing grafts in Prnp0/0 mice is that PrPC-producing tissue may induce an immune response to PrPC (43), this in turn could lead to neutralization of infectivity. Indeed, analysis of sera from grafted mice revealed significant anti-PrPC antibody titers (42). It was shown that PrPC, presented by the intracerebral graft (rather than the inoculum or graft-borne PrPSc), was the offending antigen. In order to definitively rule out the possibility that prion transport was disabled by a neutralizing immune response, these experiments were repeated in mice tolerant to PrPC, namely the Prnp0/0 mice transgenic for the PrPC coding sequence under the control of the lck-promoter. These mice overexpress PrPC on T-lymphocytes, but were resistant to scrapie and did not replicate prions in brain, spleen, and thymus after intraperitoneal inoculation with scrapie prions (44). Upon grafting with PrPC-overexpressing neuroectoderm, these mice do not develop antibodies to PrPC, presumably because of clonal deletion of PrPC immunoreactive lymphocytes. The results obtained from the previous experiments were confirmed. Intraocular inoculation with prions did not provoke scrapie in the graft, supporting the conclusion that lack of PrPC, rather than immune response to PrPC, prevented prion spread (42). Therefore, PrPC appears to be necessary for the spread of prions along the retinal projections and within the CNS.
The conclusion that can be drawn from these results is that intracerebral spread of prions is based on a PrPC -paved chain of cells, perhaps because they are capable of supporting prion replication. When such a chain is interrupted by interposed cells that lack PrPC, as in the case described here, the transport of infectivity to the target tissue is impaired. One possible explanation for this is that prions require PrPC for propagation across synapses: PrPC is present in the synaptic region (45), and certain synaptic properties are altered in Prnp0/0 mice (6,46). Another possibility is that transport of prions within (or on the surface of) neuronal processes occurs in a PrPC-dependent fashion, through conversion of PrPC by adjacent PrPSc. In this mode of transport, infectivity moves along PrPC-expressing tissue per continuitatem in a domino-stone-like manner (47).
Mechanisms of Prion Spread from Extracerebral Sites to the Central Nervous System
Even though intracerebral inoculation of prions is the most efficient way of transmitting prion diseases, from an epidemiological point of view oral uptake of prions may be more relevant than intracerebral transmission, because this way of prion uptake is thought to be responsible for the bovine spongiform encephalopathy epidemic and its transmission to a variety of species, including humans (48,49). Prions can find their way through the body to the brain of their host, yet histopathological changes have not been identified in organs other than the CNS. Upon extracerebral infection with prions, a constant feature is the long incubation time until the development of clinical disease, which may be explained by multiplication of prions in reservoirs. One possible candidate for such a reservoir is the lymphoreticular system (LRS). This is supported by the finding that prion replication in lymphoid organs always precedes prion replication in the CNS, even if infectivity is administered intracerebrally (Fig. 2; 50). Prions may multiply silently in reservoirs during the incubation time of the disease. Infectivity can accumulate in all components of the LRS, including lymph nodes and intestinal Peyer’s patches, where prions replicate almost immediately after oral administration of prions to mice (51). Recently, it was shown that variant Creutzfeldt Jakob disease prions accumulate in the lymphoid tissue of tonsils in such large amounts that PrPSc can easily be detected with antibodies on histological sections (52).
A wealth of early studies point to the importance of prion replication in lymphoid organs yet little is known about which cells support prion propagation in the LRS. Whole-body ionizing radiation studies in mice (53), after intraperitoneal infection, have suggested that the critical cells are long-lived. The follicular dendritic cell (FDC) would be a prime candidate, and indeed PrPSc accumulates in such cells of wild type and nude mice (which have a selective T-cell defect) (54). In addition, when mice with severe combined immunodeficiency (SCID), whose FDCs are thought to be functionally impaired, are challenged with the scrapie agent intraperitoneally, they do not develop the disease, nor is there any replication of prions in the spleen (55). Further support that the FDC are essential for the replication of prions in the LRS came from a study in which chimeric mice, with a mismatch in the PrPC status between FDC and other cells of the immune system, were generated.
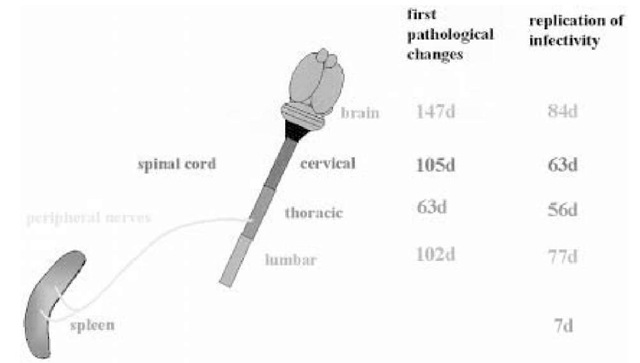
Fig. 2. Detection of PrPSc or detection of pathological changes in the spleen, the spinal cord (first number) and detection of replication of the infectious agent (second number), in the spleen, the spinal cord, and the CNS of mice, following intraperitoneal administration of the infectious agent. Infectivity and PrPSc can be detected in the LRS at early time-points. The first pathological changes and detection of infectivity within the CNS can be observed in the thoracic spinal cord.
That study demonstrated that replication of prions in the spleen depends on PrPC-expressing FDCs (56). Upon reconstitution of SCID mice with wild-type spleen cells susceptibility to scrapie is restored after peripheral infection (57). These findings suggest that components of the immune system are required for efficient transfer of prions from the site of peripheral infection to the CNS.
To study the role of the immune system in more detail, we used a panel of immune-deficient mice that were inoculated intraperitoneally with prions. Defects in the T-cell lineage had no apparent effect, but all mutations that disrupted the differentiation of B-cells prevented the development of clinical scrapie (58). From these results, one can conclude that B-cells are important for the development of scrapie, after peripheral infection. Do B-cells physically transport prions all the way from the periphery to the CNS? This possibility seems unlikely, since lymphocytes do not normally cross the blood-brain barrier unless they have a specific reason to do so (e.g., during an inflammatory reaction). Furthermore, PrPSc could be demonstrated in up to 30% of B-cell-deficient mice without any signs of clinical disease (59). How is the spread of prions accomplished within the body? Perhaps, prions administered to peripheral sites are first brought to lymphatic organs by mobile immune cells, such as B-cells. Once infection has been established in the LRS, prions invade peripheral nerve endings and find access to the CNS (60,61).
Role of B-Lymphocytes in Neuroinvasion
The replication of prions (13) and their transport from the periphery to the CNS (62) are dependent on expression of PrPC. With respect to the results described in the previous paragraph, we examined whether expression of PrPC by B-cells was necessary to support neuroinvasion. In order to study this matter, the LRSs of mice with various immune defects were repopulated by adoptive transfer of hematopoietic stem cells that expressed or lacked expression of PrPc.
Adoptive transfer of either Prnp+/+ or Prnp0/0 fetal liver cells (FLCs) induced formation of germinal centers in spleens of recipient mice and differentiation of FDCs, as visualized by staining with antibody FDC-M1 (63). However, no FDCs were found in B- and T-cell deficient mice reconstituted with FLCs from pMT embryos (B-cell-deficient), consistent with the notion that B-cells, or products thereof, are required for FDC maturation.
Mice reconstituted in the fashion explained above were challenged intraperitoneally with scrapie prions. All mice that received FLCs of either genotype, Prnp+/+ or Prnp0/0, from immunocompetent donors, succumbed to scrapie after inoculation with a high dose of prions, and most mice after a low dose. Susceptibility to disease could not be restored upon transfer of FLCs from pMT donors; omission of the adoptive transfer procedure, did not restore susceptibility to disease in any of the immune-deficient mice challenged with the low dose of prions. With the high-dose inoculum, susceptibility to scrapie could be restored, even in the absence of B-cells and FDCs. B and T cell deficient mice reconstituted with bone marrow from mice that lack T cells except those expressing TCRa/ but have intact B cells — regained susceptibility to scrapie, again confirming the dependency of infectibility on the presence of B-cells. When individual samples of brain and spleen from the scrapie-inoculated bone marrow chimeras were transmitted into highly susceptible indicator mice, we observed restoration of infectious titers and PrPSc deposition in spleens and brains of recipient mice either carrying Prnp+/+ or Prnp0/0 donor cells (63).
B-cells are clearly a cofactor in peripheral prion pathogenesis, but the identity of those cells in which prions actually replicate within lymphatic organs is uncertain. In a further step to clarify this issue, we investigated whether splenic PrPSc was associated with FDCs in repopulated mice. Double-color immunofluorescence confocal microscopy revealed deposits of PrPc-immunoreactive material in germinal centers, which appeared mostly colocalized with the folli-cular dendritic network in spleens of reconstituted mice.
Taken together, all the information we have gained with the above-described experiments support the hypothesis that cells whose maturation depends on B-cells are responsible for accumulation of prions in lymphoid tissue, such as the spleen. FDCs, although their origin remains obscure, are a likely candidate for the site of prion replication, because their maturation correlates with the presence of B-cells and their products.
Role of the Peripheral Nervous System in Prion Neuroinvasion
The question of whether accumulation of prions in the LRS is necessary or not, in order to obtain neuroinvasion, is still under discussion. There is substantial evidence for both lines of argumentation. Splenectomy prolongs the incubation time, and the key role of the LRS, including the FDC, in neuroinvasion clearly speaks in favor of an essential role of the LRS in neuroinvasion (56,58,60). On the other hand, several studies have shown that neuroinvasion can be achieved in mice devoid of an intact immune system, or in mice without PrPC expression on cells of the LRS (57,64). One argument, which speaks in favor of the essential role of the LRS in neuroinvasion, is that this system could represent the reservoir responsible for the long incubation times until the onset of clinical disease. Yet, it is also conceivable that the reservoir could be constituted by a part of the peripheral nervous system (PNS). It was shown that PrPSc is detectable in enteric ganglia after oral infection of hamsters with the scrapie agent (65). In a different experimental setup, PrPSc was detectable after intraperito-neal infection of hamsters and sheep in ganglia belonging to the autonomous nervous system, such as enteric and dorsal root ganglia (66).
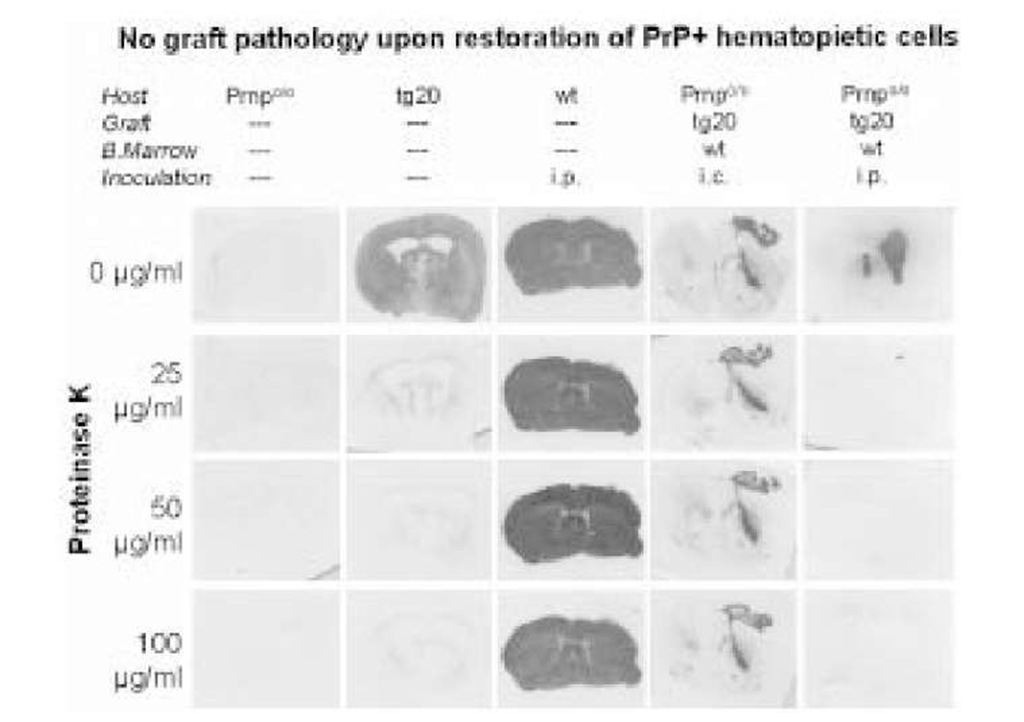
One important question is the function of PrPC in the process of neuroinvasion. Indirect evidence points to a crucial role for PrPC expression on the PNS in neuroinvasion via the PNS. PrPC-expressing neurografts in Prnp0/0 mice do not develop scrapie histopathology after intraperitoneal or intravenous iv inoculation with prions, and no infectivity is detectable in spleens. Following reconstitution of the host lymphohemopoietic system with PrPC-expressing cells, prion titers in the spleen are restored to wild-type levels but PrPC-expressing grafts fail to develop scrapie upon intraperitoneal or intra venous infection with prions (Fig. 3; 62).
In order to study the role of the PNS, and especially the function of PrPC expression on the PNS, we have developed a method to express genes of interest in the PNS. This system is used to express PrPC selectively in the sciatic nerve of a PrPC knockout mouse (67). With this system, one should be able to answer some of the open questions concerning neuroinvasion and the role of PrPC expression in the PNS.
Fig. 3. Accumulation of PrPSc in brain grafts. Histoblots showing immunoactive PrPc in brain sections natively (first row) and after digestion with increasing levels of proteinase K (PK) (second through fourth row). Prnp% mice (first column) show no immunoreactivity; mock-inoculated tg20 mice (which overexpress PrPC) show PK-sensitive PrPC (second column), but no PK-resistant PrPSc. Terminally sick scrapie-nfected wilde-type mice contain large amounts of both PrPC and PrPSc (third column). Prnp%, whose bone marrow has been reconstituted with wild-type FLCs, accumulate PrPSc in their PrPC, overexpressing grafts after ic (fourth column), but not after ip prion administration (fifth column).
Even if the accumulation of prions within the LRS is essential to achieve neuroinvasion, one common pathway for neuroinvasion could be via the PNS. How the transfer of infectivity from cells belonging to the LRS to peripheral nerves is accomplished is still a matter of discussion. Access to peripheral nerves is facilitated if myelination of the nerves is reduced or absent (68). Therefore, the mantle zone of lymph follicles, which are innervated by terminal unmyelinated nerve fibers, could be the entry point of the scrapie agent into the PNS. Possibly, this is a region where processes belonging to FDC could be in close contact with nerve fibers. Once invasion of the PNS has taken place, the agent probably travels along the peripheral nerves to the CNS. The exact mode of transport within the PNS remains to be discovered: Axonal and nonaxonal modes of transport are conceivable: For PrPC, transport in the fast axonal pathway was shown (69); for PrPSc, the mode of transport has only been studied in an indirect fashion, by comparing the incubation times of mice inoculated intraneurally to mice that were inoculated extraneurally or intrac-erebrally. In the case of intraneural injection of the agent, transport of the scrapie agent to the CNS occurred faster than in extraneurally injected mice. The actual rate of spread within the PNS was calculated to be around 1-2 mm/d (68). Obviously, this rate of spread does not correspond to the fast axonal transport. Recently, data was presented showing that PrPSc localizes adaxonally within the PNS of intraperitoneally infected hamsters and sheep: this speaks in favor of a nonaxonal transport mechanism within the PNS (66). Considering the advances in visualization of axonal and nonaxonal transport mechanisms, clarification of the exact transport mechanism of PrPSc should be possible in the near future.
Based on the assumption that prions are transported in the PNS, an important question relates to the anatomical identity of the nerves in which transport occurs. In the case of direct intraneural injection of prions, transport of infec-tivity to the CNS is accomplished via the injected nerve. Following intraperitoneal or oral infection, prions accumulate in lymphatic organs, which are predominantly innervated by nerve fibers belonging to the sympathetic nervous system (SNS). The first hints that the scrapie agent may invade the CNS using nerve fibers of the SNS came from studies aimed at unraveling the dynamics of vacuolation and replication in the CNS. Following peripheral inoculation of the scrapie agent, the first pathological changes, such as spon-giosis, as well as replication of the infectious agent, appear in the midthoracic spinal cord, in the same level where nerves from the SNS enter the spinal cord (Fig. 2; 70,71). More recent studies describe an additional access route that by passes the spinal cord, using nerves of the parasympathetic nervous system, namely, the vagal nerve. This alternative route seems to be highly significant when animals are challenged via the oral route of infection (72,73).
Conclusion
Peripheral pathogenesis of prion diseases is here defined as the process starting with the contact of the infectious agent with extracerebral sites, and eventually resulting in brain disease. This process occurs in distinct sequential phases. The earliest event in disease progression is certainly accumulation of prions in the LRS. This process is dependent on components of the host immune system. Whether prions replicate, or merely accumulate in the LRS is not known with certainty. FDC play a major role in this process, but the details are still under discussion. In order to achieve efficient neuroinvasion, either B-cells per se, or their products, are essential. One B-cell-dependent event that is of relevance is the acquisition of a functional FDC network within the germinal centers of peripheral lymphoid tissue.
The second phase of neuroinvasion appears to encompass transfer of prions from lymphoid tissue to nerve endings of the PNS. Because lymphoid organs are predominantly innervated by nerve fibers of the SNS, this part of the SNS is a prime candidate. How neuroinvasion is accomplished, and how the agent is transported within the PNS however are still unclear. It is worthwhile noting that the innervation of lymphoid tissue is, at least in part, controlled by lymphocytes themselves, because both T- and B-cells secrete nerve growth factor and, vice versa nerve terminals secrete a variety of factors that stimulate the immune system (74). These factors may play a critical role in the neuroinvasion process and represent a critical site for modulation of disease progression. For example, drugs that act on lymphocytes or on the sympathetic innervation of lymphoid tissue, or those that prevent cytokine release or block neurotransmis-sion, may have a strong influence in the immune modulation, and may represent useful tools for studying the cellular and molecular basis of prion neuroinvasion.
![Noninfected and scrapie-infected neural grafts in brains of Prnp0/0 mice. Upper row (A,B) Healthy control graft 230 d after mock inoculation. The graft is located in the third ventricle of the recipient mouse (A, see asterisks, hematoxylin and eosin), and shows no spongiform change, little gliosis (B, immunostain for glial fibrillary acidic protein [GFAP]) (C,D) Scrapie-infected graft 235 d after inoculation with increased cellularity (C), brisk gliosis (D). Bottom row: High magnification of a similar graft shows characteristic pathological changes in a chronically infected graft. (E) Appearance of large vacuoles and ballooned neurons (arrow). In the GFAP immunostain (F), astrocytes appear wrapped around densely packed neurons. Noninfected and scrapie-infected neural grafts in brains of Prnp0/0 mice. Upper row (A,B) Healthy control graft 230 d after mock inoculation. The graft is located in the third ventricle of the recipient mouse (A, see asterisks, hematoxylin and eosin), and shows no spongiform change, little gliosis (B, immunostain for glial fibrillary acidic protein [GFAP]) (C,D) Scrapie-infected graft 235 d after inoculation with increased cellularity (C), brisk gliosis (D). Bottom row: High magnification of a similar graft shows characteristic pathological changes in a chronically infected graft. (E) Appearance of large vacuoles and ballooned neurons (arrow). In the GFAP immunostain (F), astrocytes appear wrapped around densely packed neurons.](http://what-when-how.com/wp-content/uploads/2011/08/tmpD27_thumb_thumb.jpg)