Loss of Function
Despite the clear role of glia in cell culture models of PrP106-126 toxicity, the foregoing discussion indicates that glial effects, although necessary, are not sufficient for induction of neuronal loss. The foremost indication for this is the necessity for PrPC expression. Neither PrP106-126 nor PrPSc was toxic to cultures of cerebellar cells from PrPC-deficient mice (24,49). Combining PrPC-deficient neurons with PrPC-expressing microglia did not alter the result: PrP106-126 was not toxic to these cells in this situation. As described above, combining PrPC-deficient neurons with PrPC-expressing astrocytes did create a system in which PrP106-126 was toxic, but this effect was dependent on the presence of glutamate. Furthermore, this latter experiment does not explain how PrP106-126 is toxic to wild-type cerebellar cell cultures.
Treatment of wild-type neurons or PC12 cells with PrP106-126 leads to a drop in cellular resistance to oxidative stress (13,48,54). This implies that cerebellar cells are more prone to the toxicity of oxidants, such as superoxide. PrPC-deficient neurons do not show an increased sensitivity to oxidants in the presence of PrP106-126. Despite this, there is evidence that PrP106-126 alters the metabolism of PrPC-deficient cells (67). However, phenotypically, neurons and astrocytes (13,51) from PrPC-deficient mice are more susceptible to oxidative stress, compared to wild-type cells. Spontaneous apoptosis of granular cells in cerebellar cell cultures occurs at a higher rate within the first 2 d of culture than in wild-type cultures. This difference can be blocked with antioxidants applied to the culture (48), or by transfection of the cells with either PrPC or Bcl-2 (68). Increased expression of PrPC in PC12 cells is linked to an increase resistance to oxidative stress and copper toxicity (48).
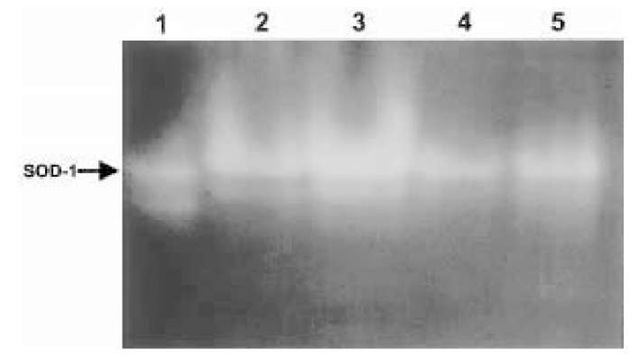
The effect of PrP106-126 on the activity of various antioxidant proteins was also examined to determine why neurons become more sensitive to oxidative stress when treated with PrP106-126. Cerebellar cells treated with PrP106-126 showed decreased activity of the enzyme Cu/Zn SOD1 (48,69), a cytoplasmic protein involved in breaking down superoxide (Fig. 4). Other enzymes, such as glutathione peroxidase and catalase, showed no sign of change in activity.
Once again, there is a parallel between cells treated with PrP106-126 and cells from mice deficient in PrPC expression and PrPC activity. Extracts from cerebellar cells and brains of PrPC-deficient mice also had lower levels of SOD activity (48). Further analysis of the phenotype of PrPC-deficient mice indicated that this reduction in activity was not caused by downregulation of superoxide dismutase expression but was due to decreased incorporation of copper into the molecule (14). Despite this, PrP106-126 also directly inhibited the activity of Cu/Zn superoxide dismutase (48).
The parallels between the phenotype of PrPC-deficient cells and cells treated with PrP106-126 suggest that PrP106-126 may induce a loss of function. This would suggest that PrP106-126 can directly inhibit the function of PrPC. Recent work indicates that PrPC itself is an antioxidant enzyme (12). However, there is no clear evidence yet that PrP106-126 directly inhibits this activity. However, there is sufficient evidence to suggest PrP106-126 impairs neuronal resistance to oxidative stress. Furthermore, studies on scrapie-infected mice have found evidence of oxidative damage and mitochondrial disfunction indicative of oxidative stress (70). These findings give credence to the results from cell culture studies with PrP106-126.
Summary
The mechanism of toxicity of PrP106-126 has been examined in detail. Although some gaps in the knowledge of its action are still present, there is sufficient evidence to create a theory of its action. This theory can also be assessed by observing what is known about neurodegeneration in models such as mouse scrapie.
Fig. 4. The activity of Cu/Zn superoxide dimutase (SOD-1), from extracts of cells treated with PrP106-126, was determined. This assay is based on protein electrophore-sis in a nondenaturing acrylamide gel. After electrophoresis, the protein bands corresponding to SOD1 can be visualized in the gel, by exposing them to a source of oxygen radicals and nitro blue tetrazolium, which produces a blue color when the radicals are detected. Clear bands represent the activity of SOD. Lane 1, pure bovine SOD; lanes 2-5 are samples from wild-type cerebellar cells treated with either vehicle (2), xanthine oxidase (3), or 80 yM PrP106-126 (4) or 20 yM (PrP106-126) for 1 d. Xanthine oxidase, a source of oxidative stress, causes an increase in the intensity of the bands, corresponding to SOD-1 activity. PrP106-126 causes a reduction in activity.
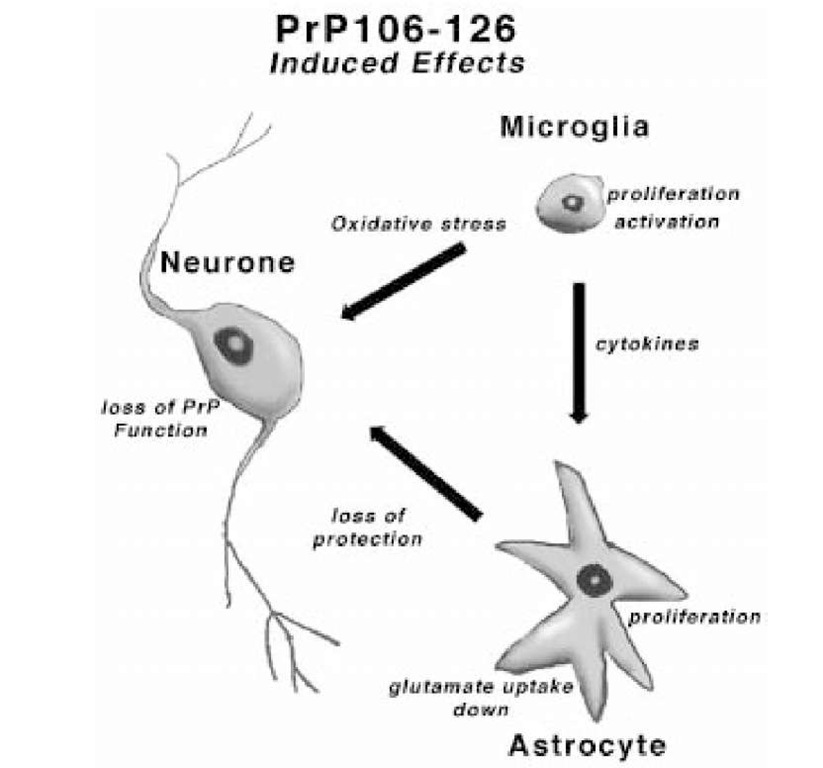
A schematic representation of PrP106-126 toxicity is shown in Figure 5. PrP106-126 aggregates to form fibrils. This peptide is capable of binding to proteins on the surface of different cell types, and can be internalized (55). PrP106-125 is toxic to neuronal cultures containing a mixture of other cells, such as micro-glia and astrocytes. For this toxicity to occur, PrP106-126 must interact with neurons and glia. PrP106-126 activates microglia, causing them to release superoxide and cytokines. The cytokines initiate astrocytic proliferation. PrP106-126 interacts with astrocytes, priming them to respond to mitogens from microglia and inhibiting glutamate uptake. The direct effects of PrP106-126 on neurons blocks the activity of PrPC, and this directly or indirectly leads to diminished protection of the injured neurons to oxidative stress. In this susceptible state, PrP106-126 treated neurons are assaulted by toxic substances in the environment, such as superoxide and glutamate which initiates signal transduction cascades, leading to increased uptake of calcium (50) and ending in neuronal apoptosis. Further investigation of animal paradigms, such as mouse scrapie, will determine if this in vitro model is relevant to true prion disease.
Fig. 5. Summary figure showing the details of the theoretical toxic mechanism of PrP106-126 to neurons, as described in the text.