Very little is known about the specifics of intrauterine growth and development in cetaceans. Indeed, the precise time intervals of such development, the basic genetic determiners, and any distinctive growth trajectories are basically unknown. What is known about cetacean prenatal development is that, as they are mammals, it is to be expected that the same basic stages of early cell division, pattern formation, organogenesis, and growth and differentiation will also be similar. For example, the “embryonic” period is usually defined as the time frame within which an animal’s body plan and its organs and organ systems (i.e., integument, skeletal, muscular, nervous, circulatory, respiratory, digestive, urinary and reproductive) are established. Once all organs form, the “fetal” period of growth and distinctive development commences. Cetacean prenatal development will similarly follow this course. It is also to be expected that the absolute time of these periods will differ from terrestrial species, between odontocetes and mysticetes, and among the different species therein.
Many studies that have noted aspects of cetacean prenatal development (most in passing rather than by detailed, systemic analysis) have used terms such as “embryo” or “fetus” in a seemingly imprecise manner. It is, indeed, often difficult to determine from these whether the authors (a) are cognizant of the biological difference between an embryo and a fetus (many reporters are not anatomists or embryologists) or (b) are able to discern differences that afford the distinction to be made with any degree of accuracy. Adding to this complexity is the fact that the precise gestation periods for many cetacean species are not known. In light of these observations, our use of the terms embryo and fetus (or embryonic and fetal periods) should be taken as representing approximate guides to stages of development rather than as a precise descriptor of an absolute time frame.
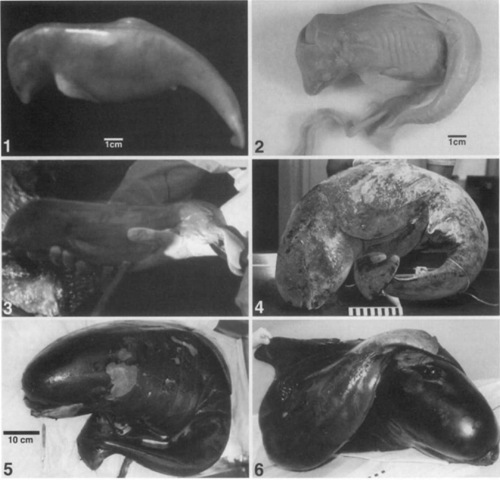
It is important to remember in discussing cetacean prenatal development that most current knowledge derives from observations on embryonic or fetal specimens discovered in pregnant cetaceans either found stranded or taken aboard whaling ships. In many cases, only a length or weight is recorded (if at all) with an occasional description of external appearance. It is usually impossible to distinguish the age of the specimen, as the date of conception and length of gestation cannot be known with any certainty. As most breeding and calving seasons are known, however, some approximations are available and have been provided (Figs. 1-6).
Figures 1-6 Fetal long-finned pilot whales (Globicephala melas) obtained postmortem from pregnant, beach-stranded whales on the shores of Cape Cod, Massachusetts. The figures are arranged numerically in order of the specimens’ lengths, which should mirror the order of their gestational ages. Each black or ivhite square equals 1 cm on the rtilers in Figs. 4 and 6.
Figure 1 Afresh specimen of a very small, unpigmented fetus. Based upon its external appearance, this appears to be a very early fetus, probably very close to the transition between embryonic and fetal periods of development. Note the prominent rostrum and the rudimentary development of a dorsal fin, tail flukes, and genital tubercle. The dark spot above the mouth and anterior to the eye appears to be the left nostril.
Figure 2 An early fetus preserved in alcohol. Due to dessication, the unpigmented fetal skin (which was pink) is now discolored and shrunken against the skeleton. The fetus is curled in the fetal position, with the tail folded laterally to the left side and the dorsal fin flattened against the body. Note the attached umbilical cord and the prominent rostrum. The dorsum of the skull is depressed (from the dehydration) at the membranous fontanelle. Just anterior to this depression is the blowhole (the paired nostrils appear to have fused into one bloivhole and have migrated dorsally and near the midline).
Figure 3 Afresh fetus is being held in a pair of gloved hands. The fetus, discovered during an autopsy, is still attached by its umbilical cord to the mother whale and is partially enveloped in its thin layer of fetal membranes (note transparent membrane and vessels at vent-rum of tail stock). It has some streaks of gray pigmentation, and the melon has begun to form over the rostrum.
Figure 4 A frozen fetus still curled in its fetal position. The skin is pigmented black, but it is covered with whitish-gray deposits of frost from thaiving. Note the developing melon over the rostrum, curled tail flukes, and the skin folds (from the folded fetal position) along the concave surface of the right lateral side. The protruding penis can be seen as a conical structure with a hooklike curl at the tip.
Figure 5 A thawed, full-term fetus. The lighter patches are areas where the skin has sloughed off postmortem during the freezing. The fetus is curled in the fetal position as it was found in utero, with the tail folded against the lift side. The umbilical cord is evident under the tail stock. The left lateral surface of the thorax and abdomen shows a number of dorsoventral stripes, which indicate grooves between puckers where the skin is folded (the skin is partiadarly crimped at the beginning of the tail stock). The dorsal fin lies flat against the body and the tail flukes are curled ventrally. Note hoxv the well-developed melon now overrides the rostrum.
Figure 6 Another full-term fetus (thawed) curled in a slightly different fetal position as it was found in utero. Here the tail bends to the right side and the flukes lie over the dorsum and extend to the left side. The blowhole has migrated to the top of the head, and the bulbous form of the melon can be seen clearly. The dorsal fin, which was flattened along the right side, is being held up by a gloved hand.
I. Development of Organ Systems
A. Integument and External Characteristics
1. Skin The overall coloring of the embryo appears light pink due to the transparency of the skin (integument) allowing the underlying tissues perfused with blood to be visible. The skin consists of the epidermis (which has four layers), dermis, and hy-podermis, which increase in thickness throughout the embryonic period (Meyer et al., 1995). Skin coloration begins during the early fetal period. In mysticetes, dark coloration occurs initially along the rostrum bordering the opening of the oral cavity. As the mysticete fetus grows, dark patches appear along the dorsum of the thorax and abdomen and on the pectoral flippers, tail flukes, and dorsal fin. The separate and irregularly shaped patches fuse and grow into a more uniform pattern (for many species, this is usually a countershaded pattern of dark dorsum and light ventrum that resembles the adult’s coloration).
2. Hair Hairs can be found along the surfaces of the upper jaw. In odontocetes, hairs appear on the lateral aspect near the tip of the rostrum, whereas in mysticetes, they are found both laterally and dorsally on the broad rostrum. In some cetacean species, these hairs can also be found on the margins of the lower jaw. These hairs appear to have some tactile properties and may derive from the vibrissae of terrestrial mammals. While most odontocetes will lose these hairs shortly after birth (except perhaps platanistoids), they are retained into adulthood in some species of mysticetes.
3. External Ears External ears (pinnae) do not develop, thus maintaining a streamlined surface contour in the ear region of cetaceans. Only a remnant of the external auditory canal is visible as a small hole present in the skin behind the eye.
4. Mammary Glands Mammary glands (mammae) are epidermal organs derived from modified sweat glands. In terrestrial mammals, and presumably cetaceans, the mammae develop along a mammary ridge (the “milk line”), which extends bilaterally from the axilla (where the forelimb joins the thorax) to the inguinal region (where the hindlimb joins the pelvis). The position of the mammae that eventually develop varies in different species: thoracic (e.g., primates, sirenians), thoracoabdominal (e.g., felids), thoracoinguinal (e.g., canids, suids), and inguinal (e.g., ungulates). Like their ungulate relatives, Cetacea only develop inguinal mammae. In females, the teats (nipples) of the mammae are internalized, being withdrawn into the mammary slits (which are positioned with one on either side of the genital slit). This internal location helps streamline the body contour and thus reduce drag during locomotion.
5. Genitoanal Slit and Contents It is difficult to sex the cetacean embryo or early fetus. As the genital tubercle develops, however, it is directed cranially in males and caudally in females (Amasaki et al, 1989b). While the penis/clitoris may be totally exposed in an earlier fetus, the external genitalia are usually not completely visible in the full-term fetus as they are withdrawn into the genitoanal slit. (Note that in a postmortem specimen, the tip of the penis usually protrudes through the slit due to relaxation of the retractor penis muscle.) The genitoanal slit opens into a common vestibule occupied caudally by the anus and rostrally by the urogenital openings. In males, the urethra is contained in the penis; in females, the clitoris and urethra are separate, and there is an opening for the vagina. In males, the genitoanal slit is elongated, reaching almost to the navel. In comparison, the genitoanal slit of females is very short, appearing only between the two mammary slits. Both males and females have a streamlined external shape, as the penis or clitoris is withdrawn into the genitoanal slit and there is no scrotum (testes are intraabdominal) or labia, thus further reducing drag during swimming.
B. Musculoskeletal System
1. Extremities The forelimb extremities of whales are called pectoral flippers. Although cetaceans are derived from a quadrupedal ancestor, adult whales do not possess hind limbs. During the embryonic period, both fore and hind limb buds are present as paddle-shaped projections, with the forelimb developing before the hind limb (Amasaki et al, 1989c). The rudimentary hind limb buds form skeletal element anlagens, vascular plexes, and nerves (Sedmera et al, 1997a) but are completely absorbed by the fetal period. By birth, the only remaining vestige of the hind limb is a skeletal remnant of the femur embedded into the lateral body wall and a rudimentary pelvis that is not attached to the vertebrae. The forelimbs, however, continue developing during the embryonic and fetal periods. Early on, they assume the elongated shape of a typical mammalian arm and forearm, with grooves separating the dig its apparent toward the distal edge. The skin overlying the flippers matures faster than the skin over the tnink (Meyer et al., 1995). The stalk-like arm and forearm foreshorten into one functional unit. The skeletal elements (humerus, radius, ulna, and carpal bones) lose their mobility at the elbow and wrist joints, maintaining flexibility only at the shoulder joint.
During the fetal period, the manus of the pectoral flipper fuses into a leaf shape (the distal portion never separates into individual digits, and the interdigital grooves disappear). Odon-tocete flippers contain five digits—a pattern reminiscent of a terrestrial ancestry. The number of digits within the flipper varies in mysticetes: members of the Balaenidae and Esch-richtiidae families retain all five digits, whereas rorquals (members of the family Balaenopteridae) have reduced that number to four. The tip of the flipper elongates in the caudal direction as differentiation of the phalangeal cartilages progresses proxiinodistally. Central digits exhibit hyperphalangia (or polyphalangia), i.e., the number of phalangeal elements expands beyond the maximum of three found in most terrestrial mammals (Calzada and Aguilar, 1996). The degree of hyperphalangia varies greatly among species. For example, the second and third digits of Globicephala melas have 14-15 and 11 phalanges, respectively, whereas there are seven elements in each of these two digits in Stenella attenuata and only five for each of these digits in Physeter macrocephalus. Expansion in the number of phalangeal elements, rather than in the lengths of the elements, probably helps support the elongated form of the flipper while retaining some small degree of flexibility that is reminiscent of fin function in fish. Hyperphalangy and elongated pectoral flipper form may also relate functionally to the increasing/decreasing aspect ratio (i.e., relationship between length and width), hydrodynamic form (streamlining effects), or locomotor function (limited to steering, braking, and lift in most species, but can include increased maneuverability or propulsion, e.g., humpback whales).
2. Tail The tail flukes do not appear until the fetal period, after the hind limbs have regressed. The midline of the tail enlarges dorsally and ventrally in the vertical plane to form the slender and hydrodynamic tail stock. The number of caudal vertebrae may increase above that typically seen in terrestrial mammals (perhaps up to 24 in mysticetes and perhaps up to 48 in odontocetes, compared with up to 21 in ungulates). Note that the actual number of caudal vertebrae is difficult to determine with accuracy, as there are no clear anatomical landmarks to separate the caudal region from the lumbar region. The caudal tip develops two horizontal plates of tissue that do not contain any skeletal elements. These plates form the tail flukes. As the fetus nears full term, the tail flukes curl ventrally at their caudal tips so that they are directed rostromedially. This curling of the flukes makes the tail tip more compact and easier to present through the vagina during birth (see later).
3. Back, Dorsal Fin, and Ribs At about the same time that the tail flukes appear, a bulge develops along the midline of the back in the region where the dorsal fin will form. The bulge shape is then modified to a species specific shape (e.g., falcate, triangular, rounded, ridge). When sexual dimorphism in fin height is seen (e.g., Orcinus), it does not occur prenatally. The vertebrae of the back unfold from the embryonic curvature (ventrally concave) to a horizontally aligned column in the early fetal period. In the late fetal period, however, the growing fetus folds again, only this time the body curves laterally. This flexibility may be possible, in part, due to the lack of a sacrum and lengthening of the vertebral column. There are additional lumbar vertebrae in most cetaceans (perhaps up to 15 in mysticetes and perhaps up to 29 in odontocetes) compared with the usual six of ungulates or five of humans. Again, this number is difficult to determine with accuracy, as there is no sacrum or pelvis, and rib articulations can vary. As the side of the fetal head approximates the tail, the dorsal fin folds flat against the concave side of the body. Dorsal fin folding facilitates vaginal delivery (see later).
The ribs of odontocetes are hinged along the lateral aspect, giving each rib two osseous elements joined by a synovial joint. Postnatally, this will facilitate thoracic cavity collapse during diving (as pressure increases with depth, the volume of air in the lungs will decrease).
4. Head (Position and Shape) and Neck The large embryonic head lies in the typical mammalian pose with the face directed ventrally at 90° to the long axis of the body. The maxillary and mandibular regions form a ventrally projecting, conical rostrum that curves slightly caudally. This projection resembles a parrot’s beak, being rather thick at the base. In the early fetal period, the rostrum elongates, particularly in long-beaked species (e.g., Stenella longirostris, Platanista gangetica). In the midfetal period, the head and neck junction straightens into the adult position, aligned horizontally with the body. The neck region shortens and stiffens, and in many species (e.g., Globi-cephala macrorhtjnchus) most of all seven cervical vertebrae become compressed craniocaudally and fuse together (Ogden et al., 1981). This enables a smoother transition in form between the head and the thorax, and a midline head position relative to the body’s longitudinal axis. The shortened neck enhances streamlining, and fusion of cervical vertebrae improves head stability during locomotion. Vertebral fusion limits lateral or rotational head motion, leaving only dorsocaudal head movements (which help begin the propulsive body wave) at the large joint between the first cervical vertebra and the skull’s enlarged occipital condyles.
5. Hyoid Apparatus The hyoid apparatus is derived from the second and third branchial arches. The single basihyal and paired thyrohyals form the large “U”-shaped plate to which the muscles of the tongue, larynx, and sternum attach, and the paired epihvals, ceratohyals, stylohyals, and tyinpanohyals form the osseous chains bilaterally connecting the basihyal with the skull (Reidenberg and Laitman, 1994).
6. Skull (Mandible, Ear Ossicles, and Cranium) The mandible (jaw) forms around a cartilaginous precursor (Meckel’s cartilage) derived from the first branchial arch. The cetacean mandible is largely composed of a horizontal body, with very little (if any) vertical projection forming the ascending ramus. The condylar process is short, and the condylar head may appear to rest directly superior to the caudal portion of the mandibular body. In many odontocetes, the condylar head migrates with fetal development to the caudal aspect of the mandible, whereas in some mysticetes, the condylar head occupies the dorsocaudal edge of the mandible. While the ascending ramus develops most of its vertical height postnatally in many terrestrial mammals, the ascending ramus of cetaceans remains practically nonexistent through the adult stage.
The caudal portion of the first branchial arch contributes to the formation of the upper portions of the first two ear ossicles (malleus and incus). The caudal portion of the second branchial arch contributes to the lower portion of these same two ear ossicles as well as the body of the third ossicle (stapes, except for the foot plate, which derives from the otic placode).
In terrestrial mammals, and presumably cetaceans, the skull is derived from two types of bone: chondrocranial (that which preforms in cartilage and then ossifies) and desmocranial (that which does not form a cartilaginous stage, but rather ossifies directly in mesenchyme). The portion preformed in cartilage (the skull base) tends to be less plastic in its shape than that which ossifies from membranes (the cranial vault). Cetaceans appears to be no exception to this rule. In fact, they are an excellent example of the plasticity of the cranial vault, as this region is grossly modified compared with terrestrial mammals.
In the fetal period, the elements of the cranial vault begin to shift their relative positions so that the maxilla approximates or meets the occipital dorsally. This process of bony overlapping (called “telescoping”) creates a layered appearance to the skull, where portions of bone are buried on the inner surface. In odontocetes. the parietals are depressed laterally and the premaxillary and maxillary bones overlap the frontal bone dor-sally, whereas in mysticetes, the preinaxilla slides over the frontal and the base of the maxilla moves under the frontal bone. The cranial vault thus changes shape from dolichocephalic (longer than wide) to brachycephalic (wider than long). The ear ossicles begin to rotate into their adult position during the early fetal period. No paranasal air sinuses (i.e., maxillary, ethmoid, sphenoid, or frontal) form within the skull either prenatally or postnatally (a diving adaptation that prevents injury from expanding/contracting the volume of an enclosed air space during depth/pressure changes). The bony nares migrate caudally to the near adult position on the dorsum of the head.
C. Respiratory Tract
1. Nasal Tract Structures of the nasal region form in the early fetal period, but asymmetry is not detected yet. Nasal conehae (bony plates that project from the nasal septum and walls in terrestrial mammals) never form. The nasal plugs (the tissues that close off the airway) are present and may derive from the tissue that forms the upper lip in terrestrial mammals. The odontocete melon, which may also derive from this same tissue, has not yet formed the characteristic bulge in the forehead region. The nasal air sacs, diverticulae of the nasal tract, begin to bud off the soft tissues of the nasal passageways.
The nasal apertures, which appear initially on the dorsum of the rostrum, begin to migrate caudally toward the adult position at the top of the head. They can be found near the junction of the rostrum and the swelling containing the forebrain.
The nasal skull grossly transforms so that the nasal floor projects ahead of the nasal passageways into the rostrum, whereas the lateral parts (which forms the walls of the nasal passages) shift from horizontal to vertical (Klima, 1999). There are two separate bony nasal passageways in all cetaceans. The soft tissues above the skull that surround the nasal passageways are maintained as two separate tubes in mysticetes. In odontocetes, however, the two soft tissue passageways fuse near their exit at the skin into one common blowhole opening. There are further differences within odontocetes in the development of the nasal skull. Phocoena phocoena has the most posteriorly positioned nares, whereas Physeter macrocephalus has the most anteriorly positioned nares. There are additional specializations in Physeter related to the unique forehead containing the spermaceti organ, including asymmetrically sized and positioned narial openings in the skull and soft tissue pathways through the head.
2. Larynx The larynx (voice box) forms from cartilage elements of the fourth through sixth branchial arches. Its position in cetaceans is similar to that found in terrestrial mammals. The front part (epiglottis) overlaps its ventral surface with the dorsal surface of the soft palate, creating a bridge to channel air from the nasal region into the trachea and lungs. In odontocetes, the larynx undergoes elongation of its rostral portion, forming a “goose beak” shape that inserts into the nasal region. The epiglottis elongates during the midfetal period. The posterior cartilages (corniculates) are still shorter than the epiglottis and will not reach their full proportions (i.e., exceed the epiglottis in height) until the fetus reaches full term. The laryngeal “goose beak” of odontocetes inserts into a muscular sphincter derived from the palatopharyngeal arch of the soft palate. Postnatally, this interlock will keep the rostral opening of the larynx connected with the posterior nasal cavity. This connection imparts circumferential protection from the digestive tract, allowing air to flow between the nasal region and the lungs for sound production while prey is swallowed whole underwater. Internally, the odontocete larynx develops a midline fold (which is bifurcated in Kogia breviceps) that appears homologous to the vocal fold of terrestrial mammals (Reidenberg and Laitman, 1988). The mysticete larynx more closely resembles that of terrestrial mammals, except that there is a large and muscular sac attached ventrally in the midline. In Caperea irmrginata, this sac lies on the right side.
3. Trachea and Lungs Tracheal rings usually develop as “0″-shaped rings, unlike the “C”-shaped rings of most terrestrial mammals. A bronchus leading to the right lung develops above the carina (tracheal bifurcation). As this bronchus emerges directly from the trachea above the primary (main stem) bronchi, it is termed a “tracheal bronchus.” A right tracheal bronchus is a feature also found in the closely related artiodactyls.
The lungs mature from the embryonic glandular stage to the fetal canalicular stage (for more information on stages of lung development, see Drabek and Kooyman, 1983). Next, muscular sphincters develop around the terminal bronchioles. Because this feature is not found in terrestrial mammals, it may be an adaptation for diving. The next phase of development is the alveolar stage. By the midfetal period, cartilaginous rings develop in the terminal bronchioles. This is another feature not found in terrestrial mammals that may also be an adaptation for diving, as cartilage rings may keep the terminal airways patent under high pressures and during lung collapse at depth.
D. Digestive Tract
1. Teeth and Baleen Late in the fetal period, both odontocetes and mysticetes form tooth buds. Odontocete teeth are single cusped and usually conical in shape with species-specific variations, e.g., narwhal, Monodon monoceros (long spiral tusk), Platanista gangetica (needle-shaped), beaked whales (flattened and broad), and porpoises (flattened, spade-shaped). Postnatally, teeth are used primarily for grasping and aggression. As there are no incisors, canines, or molars (odontocetes are “ho-modonts”—all teeth have the same shape), the task of breaking up food is passed onto the stomach (see later). The tooth buds of fetal mysticetes are sometimes multicusped, resembling the teeth of related terrestrial mammals (Slijper, 1979). The mysticete tooth buds are more numerous in the upper jaw than in the lower jaw, but all are usually resorbed before birth. The formation of rudimentary baleen plates, which occurs concurrently with tooth bud degradation, may be induced by the process of tooth bud resorption (Ishikawa and Amasaki, 1995). As the mysticete fetus grows, longitudinal baleen ridges form in the gums of the upper jaw. These longitudinal ridges develop transverse divisions and rows of papillae composed of epidermal folds that become comified. The cornified papillae are tubular in shape and elongate and coalesce with their neighbors to form baleen plates (Slijper, 1979).
2. Throat Grooves Throat grooves are a series of parallel, longitudinal folds found on the external, ventral surfaces of the head in rorqual mysticetes that enable expansion of the oral cavity. In other mysticetes and some odontocetes, a single pair of throat grooves can be found at the base of the jaw and may indicate expansion of this region during tongue and hyoid depression (see later). In rorqual mysticetes, throat grooves begin forming in the fetal period, appearing initially between the umbilicus and the pectoral flipper. A second set of ventral throat grooves appears next near the tip of the mandible. Toward the end of gestation, the two sets of throat grooves join to form one complete set running from the mandibular tip to the umbilicus. These throat grooves enable expansion of the floor of the mouth to engulf prey during feeding.
3. Tongue In both mysticetes and odontocetes, lingual papillae develop along the lateral border of the tongue during the fetal period. Because newborn cetaceans lack lips, these papillae probably play an important role postnatally during nursing in grasping the teat, creating a seal for suction, and forming a channel for milk to flow into the oral cavity. These papillae attain maximal size in the early postnatal period of odontocetes, but can sometimes be found persisting in adults.
The tongues of mysticetes and odontocetes differ greatly in their construction, and this difference is evident in the fetal stage. Odontocete tongues are related more closely to the tongue of terrestrial mammals, being very muscular. Their tongues have large insertions on the broad bones of the hyoid apparatus (Reidenberg and Laitman, 1994). This arrangement helps depress the tongue into the throat like a piston, thereby creating enough negative pressure to draw in prey—a mechanism relerred to as “suction feeding.” The mysticete tongue (particularly in rorqual whales) is unusual in its structure because it can be flattened against the floor of the mouth and expanded laterally along with the throat pleats during prey engulfing. In addition, there is a librocartilage structure in the ventral throat region ol rorqual whales that may be related to attachment of the mylohyoid muscle. This structure may aid jaw mechanics and support the tongue and floor of the mouth during expansion/contraction of the throat grooves.
4. Gastrointestinal Tract In mammals, gut development begins with a single, relatively straight gastrointestinal tube that is suspended in the midline of the coelom. As die embryo develops, die gut tube differentiates into the foregut and hindgut, and as each section further develops its specific shape, individual regions of the gut tube begin to rotate into different positions within the coelomic cavity. Toward the end of the embryonic period, the thoracoabdominal wall is distended. This is probably because the stomach is developing its multiple chambers and intestinal rotation is occurring. Cetaceans develop a multichambered stomach [e.g., see Tarpley et al. (1997) on die stomach of Balaena int/s-ticetus], much like that found in closely related ruminant artio-dactyls, the closest group of living land mammals to the cetaceans. The divisions of die cetacean stomach include, from proximal to distal: forestomach, main stomach, and pyloric stomach. As it does in ruminants, the cetacean forestomach arises Irom the stomach bud rather than die esophagus (Amasaki et al, 1989a). but is not divided into die three small chambers (rumen, reticulum, psalterium) found in. for example, die cow. The size ol the forestomach may be dependent on the consistency of the prev. In odontocetes, a large and muscular compartment may signify a function in breaking down whole fish or crustaceans, whereas a smaller compartment possibly relates to a diet of soft prey such as cephalopods. In mysticetes. the lorestomach is smaller than die main stomach, perhaps relating to die relatively small size of their prey. The cetacean main stomach and pyloric stomach (which can have up to 12 chambers, e.g., beaked whales) are equivalent to die cow’s single rennet stomach (Slijper. 1979).
The process of intestinal rotation probably resembles that of other mammals, involving temporary’ herniation (protrusion) into the umbilicus, rotation and folding, and then return of the contents back to the abdomen where they lie more compactly. Thus, by the early fetal period the abdomen is no longer distended by the process ol intestinal rotation. The cecum and large intestines then differentiate further, developing circular folds that divide the intestines into multiple connected chambers resembling the haustra (sacculations) of terrestrial mammals (Amasaki et al, 1989a).
E. Genitourinary System
1. Urinary Tract In embryos of terrestrial mammals, and presumably cetaceans, the earliest kidney is the mesonephros. composed ol ducts and tubules. The embryonic metanephric duct, which buds of! the mesonephric duct, becomes the ureter. As the mesonephros regresses, a second kidney structure, the metanephros. develops around the metanephric duct and is retained as the final kidney.
The fetal kidney develops as a cluster of many small and relatively independent kidney units called renicules. which will be retained in the cetacean adult. An adult kidney divided into renicules or lobes is not unusual in mammals (e.g., ox, otter) and may indicate persistence of the letal condition. The apparent Junctional advantage ol a kidney divided into renicules in large mammals appears to be related to a maximum size lor the length of the tubules, which might otherwise be too long lor proper function in a large single kidney.
The urogenital sinus (derived from the embryonic cloaca) becomes the urethra. The urinary bladder develops from the proximal portion of the allantois.
2. Reproductive Tract The remnants of the mesonephric duct become the efferent ductules, epididymis, and deferent duct for sperm transport in males. The gonads (ovaries and testes) develop from gonadal ridges, which are paired thickenings of the coelomic epithelium. In females, paramesonephric (Mullerian) ducts develop simultaneously with mesonephric ducts. Paramesonephric ducts become the bicornuate uterus and oviducts in females, but degenerate in males except for the prostatic sinus.
In males, the testes are intraabdominal, i.e., they do not descend as in most terrestrial mammals, and thus there is no scrotal sac. The internalization of these structures helps streamline the body shape, thus reducing drag during swimming. Interestingly. cetaceans develop a gubernaculum (which functions in testicular descent in terrestrial mammals), but do not develop the peritonea] outpocketing that occurs with testicular descent (the vaginal process) during the fetal period (van der Schoot, 1995). A complex vascular plexus supplies the testes (see later), functioning as a countercurrent heat exchanger to keep the testes cool despite their internal location under the insulating blubber.
The genital tubercle gives rise to the penis or clitoris (Amasaki et al, 1989b). External genitalia are not usually visible externally in the lull-term fetus as they are withdrawn into the genital slit (see Section I.A.5).
F. Circulatory System
1. Heart During the embryonic period, the heart is visible and has probably undergone a similar differentiation as occurs in other mammals. The heart begins as a straight tube, but during the late embryonic period, it lolds and forms septa that eventually divide it into the four chambers found in all mammalian hearts. The cetacean heart, however, shows diflerences in shape Irom terrestrial mammals. In both odontocetes and mysticetes, it is laterally (transversely) broad and craniocau-dallv compressed, with the apex being formed by both ventricles. The cetacean heart has specializations that may be adaptive for diving, such as anastomoses between dorsal and ventral interventricular arteries, and hypertrophy of the right ventricle (Tarpley et al, 1997). Diving adaptations also occur in the great vessels, such as an expandable aortic arch.
2. Blood Vessels The internal carotid artery, which is a major supplier of blood to the brain in terrestrial mammals, tapers dramatically in the neck and terminates under the skull base at the carotid canal before reaching the brain. This reduction of the internal carotid artery probably occurs in all cetaceans that exhibit cervical rete mirabila (see later), as this is the only structure it appeal’s to supply. Interestingly, the internal carotid artery is also reduced or absent in many artiodactyl species. The ductus arteriosus (a fetal vascular connection between the aorta and the pulmonary artery) was thought to remain patent postnatally, but a study in adult pilot whales (Globicephala spp.) showed that it does close (Johansen et al, 1988). This is no different from terrestrial mammals and is probably the condition in other cetaceans as well.
The fetus develops complex networks of anastomosing, coiled blood vessels called retia mirabila. These vessel masses are found in regions surrounding the dorsal thoracic cage, the region near the foramen magnum, and the spinal cord. Although the function of retia mirabila is not known, it is thought that they are adaptations to diving and resurfacing. Their vessel structure may compensate for the rapid pressure changes of descent and ascent with a slow and sustained response that moderates blood flow. By dampening oscillations in blood pressure, sensitive tissue such as heart muscle or the brain and spinal cord continue to receive steady perfusion, thus avoiding oxygen debt and lactic acid buildup. As these vessels appear to store blood near vital tissue (e.g., brain, spinal cord, heart), they may thus function as a reservoir, distributing blood to these oxygen-sensitive tissues when normal circulation is affected (e.g., as pressure increases during diving, or metabolism is slowed). A less widely held hypothesis for the function of the retia mirabila is in trapping the nitrogen bubbles (emboli), which may come out of solution in the bloodstream during ascent from a prolonged dive. These bubbles are potentially fatal, as they can block smaller blood vessels and therefore interrupt blood flow in the capillary beds of organs (a condition known in human divers as decompression sickness, or caisson disease).
The fetus also develops a complex network of vessels that supply and drain the testes and uterus. These vessels are arranged in a plexus to effect a thermoregulatory countercur-rent exchange. This conserves heat where needed and allows extra heat to be drawn away from these organs. Thus, the male can keep the testes cool and the pregnant female can keep the fetus in the uterus from overheating, despite the internal location under the insulating blubber (Rommel et al, 1993).
G. Nervous System
1. Brain Brain development in the embryonic period resembles that of other terrestrial mammals. The brain is composed of three main sections: prosencephalon (forebrain), mesencephalon (middle brain), and rhombencephalon (hindbrain). The corticospinal tract does not develop to the same degree as terrestrial mammals, probably due to the loss of the hind limbs and the reduced role of the forelimbs in propulsion. The cochlea is enlarging, while the vestibular system is rudimentary in size— a disparity that will remain in the adult. Olfactory bulbs and nerves are present in both odontocete and mysticete embryonic brains.
The rate of brain growth and degree of encephalization differs for different species (Pirlot and Kamiya, 1975). In the early fetal period, typical cetacean features begin to develop. For example, the olfactory bulbs and nerves disappear in odontocetes. In mysticetes, however, the olfactory bulbs and nerves are retained. Because adult mysticetes retain olfactory mucosa, it is presumed that they use a sense of smell to help locate plankton, particularly swarms of krill. There is some dispute, however, as to the existence of a vomeronasal organ (a chemore-ceptive organ that functions mainly in detecting sexual pher-mones in terrestrial mammals). Although it had been thought that adult whales had a vomeronasal organ, fetal studies of mysticetes and odontocetes show both the vomeronasal organ and nerve to be absent (Oelschlager et al., 1987). The function of a cetacean vomeronasal organ is purely speculative, but may include detecting the presence and mating status of other whales and perhaps even the odor of food in the mouth. The terminal nerve (a sensory, but not chemosensory, derivative of the olfactory placode sometimes called “cranial nerve 0″) persists and may function in the autonomic innervation of intracranial arteries and mucous epithelium of the nasal air sacs.
2. Spinal Cord In the midfetal period, the head and neck regions align horizontally. During this process, the cervical section of the spinal cord, which was previously flexed ventrally, must now arch under and around the cerebellum to join the thoracic spinal cord (the dorsal aspect is thus concave). As the cervical vertebrae are compressed, much of the cervical spinal cord is contained within the skull.
II. Gestational Length
Because whales are related to terrestrial ungulates, it is not surprising that their gestation is of a similar length (see Table I). Horses, for example, have a gestation of 11 months (compared to 9 months for a human’s pregnancy and 22 months for an elephant’s gestation). Gestation in mysticetes lasts 10-13 months. The length of gestation in odontocetes, however, is more variable, ranging from 10 to 17 months. The length of the gestation is not correlated with body size (e.g., although the sperm whale is the largest odontocete, its gestation is the same as the smaller pilot whale and less than the Baird’s beaked whale, Berardius bairdii).
The length of gestation in cetaceans may be related to food supply and migration. Most mysticetes mate in warm waters, migrate to cold waters to feed, and then migrate back to warm waters to calve. This behavioral cycle, which takes 1 year, thus appears to be related to gestational length. Interestingly, because most feeding occurs in colder waters, a pregnant mysticete whale may well be fasting while simultaneously spending energy in migratory locomotion and nourishing a growing embryo/fetus. In this regard, it is significant that the first half of the pregnancy (largely embryo development) takes place during the migration to the feeding areas, whereas the second half of the pregnancy (largely fetal growth) takes place during the migration back to the calving areas. A whale migrating to feeding areas is not carrying a large load of stored energy compared with a whale returning to calving areas from feeding areas. In some species, pregnant whales may increase food intake by 50-60% above normal during the last 6 months of gestation.
TABLE I
Cetacean Gestations and Newborn Calf vs Adult Measurements
|
|
Gestation |
Ncivborn calf |
Mature adult |
|
|
(in months) |
(length, weight) |
(length, iveight) |
|
Mysticetes |
|
|
|
|
Right whales fEubalaena spp.) |
12 |
4.5-6 m. 1000 kg |
12.5-17.7 m, 30,000-80.000 kg |
|
Bowhead whale (B. mysticetus) |
12-16 |
3.6-4.5 m, 1000 kg |
11.5-18 m |
|
Pyginy right whale (Caperea marginata) |
? |
2 in? |
5.47-6.45 m, 3.100-3,500 kg |
|
Gray whale (Eschrichtius robustus) |
13-13.5 |
4.5-5 m. 500-800 kg |
13-15.2 m, 14.000-35,000 kg |
|
Humpback whale (Megaptera novaeangliae) |
11-11.5 |
4—5 m, 900-1500 kg |
11.5-19 m, 25.000-48,000 kg |
|
Minke whales (Balaenoptera acutorostrata |
10 |
2.4-3 m. 300-400 kg |
6.9-10.7 m. 4000-13,500 kg |
|
and B. bemaerensis) |
|
|
|
|
Biyde’s whales (B. bnjdei, B. edeni) |
11-12 |
3.95-4.3 m, 900 kg |
11.6-15.6 m, 16,000-25,000 kg |
|
Sei whale (B. borealis) |
11.5-12 |
4.5 m, 780 kg |
13-18.3 m. 20.000-25,000 kg |
|
Fin whale (B. physalus) |
11 |
6.4-6.5 m, 1750-1800 kg |
17.5-27 m, 30,000-90,000 kg |
|
Blue whale (B. tmiscuhis) |
11-12 |
7-8 in, 2000-3000 kg |
19-31 m, 100,000-200,000 kg |
|
Odontocetes |
|
|
|
|
Sperm whale (Physeter macrocephalus) |
14-16 |
3.5-4.5 m, 1000 kg |
8.3-20.5 m. 16,000-57,000 kg |
|
Pygmy sperm whale (Kogui breviceps) |
11 |
1.2 m |
2.7-3.7 m, 400 kg or less |
|
Dwarf spenn whale (K sima) |
9 |
1 m |
2.1-2.7 m, 210 kg |
|
Cuviers beaked whale (Ziphhis cavirostris) |
12 |
2.5-3 m |
5.1-7.5 m, 3,000 kg |
|
Baird’s beaked whale (Berardius bairdii) |
17 |
4.5-4.8 m |
10-12.8 in, 11.000 kg |
|
Northern bottlenosed whale (Hyperoodon ampullatus) |
12 |
3-3.5 m |
6-9.8 m |
|
Southern bottlenosed whale (H. planifrons) |
? |
2.9 m |
5.7-7.45 m |
|
Hector’s beaked whale (Mesolodon liectori) |
? |
2.1 m |
4.3-4.43 m |
|
True’s beaked whale (M. mints) |
? |
2.3 m, 136 kg |
5.1-5.3 m, 1.394 kg |
|
Gervais’ beaked whale (Al europaeus) |
? |
1.6-2.1 m, 49 kg or more |
3.7-5.2 m, 1,178 kg or more |
|
Sowerby’s beaked whale (M. bidens) |
12 |
2.4-2.7 in, 185 kg |
5.05—5.5 m |
|
Gray’s beaked whale (A/. grayi) |
? |
2.42 m |
4.74-5.64 m. 1,075-1,100 kg |
|
Hubb’s beaked whale (M. carlhubbsi) |
? |
2.5 m |
5.3 m. 1,432 kg |
|
Strap-toothed whale (M. layardii) |
? |
0.76-2.8 m |
5.8-6.2 m |
|
Blainville’s beaked whale (M. detisirostris) |
? |
1.9 m. 60 kg |
4.56-4.73 m. 1,033 kg |
|
Indian river dolphin (Platanista gangetica) |
8-12? |
0.67-0.9 m |
1.7-2.5 m. 85 kg |
|
Amazon river dolphin (boto) (Inia geoffrensis) |
9-12 |
0.75-0.8 m, 7-8 kg |
2-2.6 m. 100-160 kg |
|
Chinese river dolphin (Liptotes vexillifer) |
? |
0.57-0.95 m, 10 kg or less |
2.1-2.5 m. 125-16- kg |
|
Franciscana (Pontoporia blainvillei) |
10-11 |
0.75-0.8 m. 7.3-8.5 kg |
1.5-1.74 m, 25—53 kg |
|
Beluga whale (Delphinapterus leucas) |
14-14.5 |
1.5-1.6 m. 79-80 kg |
3-5.5 m, 400-1,500 kg |
|
Narwhal (Monodvn monoceros) |
14-15 |
1.5 m |
3.4-4.7 m |
|
Commerson’s dolphin (Cephalorhynchus commersonii) |
11-12 |
0.65-0.75 m |
1.25-1.75 m, 35-86 kg |
|
Hector’s dolphin (C. hectori) |
? |
0.5-0.7 m |
1.2-1.8 m, 50-60 kg |
|
Humpbacked dolphin (Sousa chitiensis) |
p |
0.9-1 m |
2.26-3 m, 195-284 kg |
|
Tucuxi fSotaliafuviatilis) |
10 |
? |
1.3-1.9 m, 35-40 kg |
|
Common Bottlenosed dolphin (Tursiops truncatus) |
12 |
0.9-1.3 m |
1.9-4 m, 90-650 kg |
|
Pantropical spotted dolphin (Stenella attenuata) |
11-12 |
0.8-0.89 m |
1.82-2.57 m, 119 kg or less |
|
Atlantic spotted dolphin (S. frontalis) |
? |
0.76-1.20 m |
2.3 m. 143 kg |
|
Spinner dolphin (S. longirostris) |
10-11 |
0.77-0.8 m |
1.3-2.16 m. 26.5-75 kg |
|
Striped dolphin (S. coeruleoalba) |
12-13 |
1 m |
2.16-2.4 m, 156 kg or less |
|
Short-beaked common dolphin (Delphinus delphisY |
10-11 |
0.76-0.86 m |
1.6-2.6 m, 70-135 kg |
|
Fraser’s dolphin (Lagenodelphis hosei) |
? |
0.95 m |
2.25-2.65 m. 200 kg or more |
|
White-beaked dolphin (Lagenorlujnchus albirostris) |
? |
0.95-1.6 m. 40 kg or more |
2-3 m |
|
Atlantic white-sided dolphin (L. actitus) |
10-12 |
1.08-1.22 m |
2-2.8 m |
|
Pacific white-sided dolphin (L. obliquidens) |
10-12 |
0.8-1.24 m |
1.7-2.5 m, 75-181 kg |
|
Dusky dolphin (L. obsciinis) |
11 |
0.55-0.70 mb |
1.6-2.1 m, 40-80 kg or more |
|
Northern right whale dolphin {Lissodclphis borealis) |
? |
0.8-1 m |
2-3.1 m, 115 kg |
|
Risso’s dolphin (Grampus griseus) |
13-14 |
1.2-1.5 m |
2.6-4.3 m, 500 kg |
|
Melon-headed whale (Peponocephala electra) |
9 |
1 m |
2.2-2.75 m. 160-275 kg |
|
Pygmy killer whale (Feresa attenuata) |
9 |
0.8 m |
2-2.6 m, 150-225 kg |
|
False killer whale (Pseudorca crassidens) |
11-15.5 |
1.6-1.93 m |
3.96-6 m, 1.100-2,200 kg |
|
Killer whale (Orcinus orca) |
16-17 |
2.06-2.5 m |
4.6-9.75 m, 2,600-10.500 kg |
|
Long-finned pilot whale (Globicephala melas) |
14.5-16 |
1.75-1.8 m, 70-85 kg |
3.8-6.3 m. 280-1750 kg |
|
Short-finned pilot whale (G. macrorhynchus) |
15 |
1.4-1.85 m |
3.01-7.2 m. 600-3950kg |
|
Irrawaddy dolphin (Orcaella brevirostris) |
14 |
0.9-1 m. 12.5 kg |
2.15-2.75 m, 90-150 kg |
|
Finless poipoise (Neopliocaena phocaenoides) |
11-12 |
0.6-0.9 m |
1.8-1.9 m |
|
Harbor poipoise (Phocoena phocoena) |
9-11 |
0.7-0.9 m, 5-9 kg |
1.4-2 m, 40-90 kg |
|
Spectacled poipoise (P. dioptrica) |
? |
0.46 m |
1.8-2.4 m |
|
Dall’s poipoise (Phocoenoides dalli) |
11-12 |
0.95-1 m |
1.7-2.2 m, 200 kg |
a10.09-m male North Atlantic right whale was 9055 kg when weighed intact postmortem. b0.98-m dusky dolphin calf weighed 22 kg; 0.67-m dusky dolphin fetus weighed 3.7 kg. ‘ May include data for D. capensis.
Thus, the energy demands on a whale in early pregnancy may be smaller than that of a whale in the later stages of pregnancy, when the fetus is growing at a rapid rate. In odontocetes, these energy constraints appear to have less of a temporal impact on gestational periods. This may be due, in part, to a more constant energy supply (year-round access to a food supply) for those species that migrate, or the lack of migration in other species.
Because the calf must be able to swim, see, hear, and vocalize immediately after birth, the nervous and muscular systems of the calf must be well developed. This also translates to a fairly long gestation, with as much development as possible occurring prenatally (compared with the human baby, which completes much of its neuromuscular development postnatally). The long gestation also enables calves to grow to a large size before birth, reaching approximately one-fourth to one-third of the mother’s size. Once the fetus has attained a near adult form, the most dramatic changes appear to be mainly in the overall size of the fetus. As the fetal period progresses, the growth rate increases rapidly. For example, the blue whale (Balaenaptera mtisculus) gains approximately 100 kg/day in the last 2 months alone. Large newborns are also common among the whales’ closest land relatives, the ungulates, which also have well-developed neural and muscular systems at birth. An additional advantage of a large calf is a smaller surface-to-volume ratio (which helps the calf conserve heat). Thus, because whales have relatively large calves, it is not surprising that multiple births are a rarity.
III. Maternal Uterus, Placenta, and Umbilical Cord
Cetaceans have a bicornuate uterus (two horns joined in a Y shape). The fetus usually develops in one horn (either horn for mysticetes, but most frequently the left horn for odontocetes), whereas the other horn is generally occupied by the al-lantois (one of the embryonic membranes) and placenta. The cetacean uterus has a complex vascular plexus that functions in countercurrent heat exchange (Rommel et al, 1993). This keeps the fetus from overheating, despite its insulated location under the maternal blubber and adjacent to the locomotor musculature of the maternal abdomen.
The placenta is epitheliochorial (or cotyledon), which means that the maternal and fetal tissues do not fuse into one tissue, as in humans. Rather, their vascular systems remain separated by two epithelial layers with separate capillary beds. This arrangement ensures that the two layers separate relatively easily at birth, thus minimizing the inevitable loss of blood. Not surprisingly, this type of placenta is also found in the ungulates, the group of terrestrial mammals related most closely to cetaceans.
The umbilical cord is short and thick, with “amnion pearl” knobs on the outer surface that appear to regulate the development (cornification) of fetal skin. It contains two arteries and two veins, as well as an allantoic duct. When the calf is born, the umbilical cord breaks off at the fetal end, allowing the calf to swim unhindered to the surface. Because the mother does not appear to bite off the umbilical cord, it is presumed to break with little force at the moment of birth. The umbilical ring contains invaginations that probably weaken the connection between the fetal epithelium and the umbilical cord. The umbilical arteries and veins are also constricted and weak where they enter the fetal abdomen. The umbilical cord attaches midway along the length of the fetus (unlike the more caudal attachment found in fetuses of other mammals, in which the neck contributes more to fetal length than the tail). Thus, the umbilical cord will be stretched taut to the same degree regardless of whether the head or the tail is delivered first. The stretch from the delivered fetus pulling taut the umbilical cord, which is attached via the placenta to the mother’s uterus, may cause its rupture at the umbilicus.
IV. Fetal Position and Birth
Birth takes place underwater. In most observed captive births in odontocetes, the fetal tail emerged first through the vaginal opening. This tail-first presentation may appear unusual, particularly when compared with the usual head-first presentations of most terrestrial mammals. Interestingly, captive manatees have also been observed to deliver their young tail-first underwater. Whereas births in the wild have been documented less frequently, they appear to be more commonly tail-first presentations in odontocetes and may be equally tail first or head first in mysticetes. As the pelvis in whales is rudimentary, it appears to have little, if any, effect on passage of the fetus during birth. In fact, the large size of the cetacean brain at birth may be possible, in part, because of the ease with which the large head of the fetus can be delivered through this rudimentary pelvis. Because there is no significant bony constriction at the pelvic outlet, there does not appear to be a physical need for a head-first delivery as in most large terrestrial mammals.
The higher frequency of tail-first presentations may also be explained by the shape and intrauterine position of the fetus. The cetacean fetus has a fusiform shape, with the rostrum and tail stock both being relatively small in diameter. The tail flukes, dorsal fin, and pectoral flippers are very pliable and are flattened against the body (fin and flippers) or curled back toward the midline to form a small knob (flukes). This folding and curling not only helps keep the fetal body within in the smallest dimensions, but it also enables the fetus to maintain a relatively smooth exterior contour with no protrusions to inhibit delivery through the vagina. In addition, the whole fetus is laterally flexed into a U shape, with the tail recurved toward the head so that the flukes are positioned adjacent to the rostrum. While this fetal folding reduces the intrauterine volume needed for carrying the fetus, it also leaves the fetus with both its rostrum and its tail flukes directed toward the maternal tail. However, because the uterine horn is also folded, only one end of the fetus can thus be directed toward the cervix. In odontocetes, it is most commonly the tail. Because the tail flukes are smaller than the cetacean head, they can therefore slip out of the vagina more easily and thus are more likely to emerge first.
The head being directed away from the cervix before parturition may be a function of either fetal shape or fetal weight. Because both the center of gravity and the largest diameter is closer to the fetal head, its “rest” position may more likely be with the heavier fetal head nearer the center of gravity of the mother. This places the fetal head in the more distensible part of the mothers abdomen and away from the more mobile tail stock (which, due to locomotor constraints, may have less capability for expansion).
Once the fetus is settled into this birth position, continued growth appears to cause it to recurve caudally in order to fit within the mother’s abdomen. Unlike terrestrial mammals, the head is not flexed ventrally in the late-term cetacean fetus because there is practically no neck and the cervical vertebrae are largely fused. Rather, the fetus folds in half laterally to conserve space. The curved midsection of the fetus takes up more room in the maternal abdomen than the fetal head and tail. Thus, the fetal abdomen is placed cranially in the mother, where there is more room for expansion, while the fetal head and tail are directed caudally near the less expandable maternal tail stock. Although the fetal head is directed caudally, it is positioned at the tip of the uterine horn (which is thus also folded to face caudally) and not adjacent to the cervix. In this folded position, it is unlikely that the fetus can reposition itself to completely switch from a tail-first to a head-first presentation. As the fetus is delivered, its body must unfold. Thus, midway through parturition, the fetal head will again face toward the maternal head as the fetal body straightens. The newborn calf bears light colored bands and shallow vertical grooves, called “fetal folds,” along the skin of the lateral abdomen. These markings indicate the concave side of the fetus as it was folded in utero.