Thysanoptera
The 6000 described species of Thysanoptera, the thrips, exhibit a wide range of biologies. About 50% feed only on fungi, with most of these feeding on hyphae but some on spores. Of the remainder, approximately equal numbers feed either in flowers or on green leaves; a few are obligate predators on other small arthropods. Several opportunist species are crop pests, causing feeding damage and vectoring tospoviruses, but sometimes acting as beneficials by feeding on other pest arthropods (Fig. 1D ).
Thrips have unique asymmetric mouthparts involving only one mandible, a life history that is intermediate between those of the hemi- and the holometabola, and a haplodiploid sex control system. Many thrips species exhibit complex behavioral patterns including lekking, fighting, and eusociality, and in the tropics many species induce galls on leaves.
THYSANOPTERA STRUCTURE
Thrips have characteristic feeding stylets, legs, and wings. Larvae and adults have only a left mandible, and their paired maxillary stylets (lacinia) are coadapted to form a feeding tube with a single central channel and a subterminal aperture. The legs of adults lack typical insect tarsal claws, but each tarsus has an eversible bladder-like aro-lium. The two pairs of slender wings bear fringes of long cilia. Other features include a pair of antennae with seven, eight, or nine segments, although various species have segments fused to produce lower numbers. The head usually bears two large compound eyes, reduced to less than 10 ommatidia in some wingless species, and there are three ocelli between the compound eyes.
Members of the two suborders of Thysanoptera, Tubulifera and Terebrantia, differ from each other considerably in structure. In adult Tubulifera, the forewings have a smooth surface with no longitudinal

FIGURE 1 Thysanoptera diversity. (A) Lichanothrips pulchra female, which creates a domicile by gluing together pairs of Acacia leaves. (B) Kladothrips waterhousei foundress, which induces leaf gall on Acacia. (C) K. waterhousei first-generation female, which functions as a soldier to defend a gall. (D) Western flower thrips (Frankliniella occidentalis), one of the world’s major insect pests.
veins, and the fringing cilia insert directly into the wing membrane. When at rest, the wings lie flat on top of each other on the abdominal tergites, held in place by one or two pairs of sigmoid setae. Moreover, the 10th abdominal segment is tubular, with the anus terminal but the genital opening at the base of the tube, the female’s ovipositor being an eversible, chute-like, structure.
In adult Terebrantia, the forewing surface is covered in micro-trichia, two longitudinal veins are visible, and the fringing cilia are inserted into sockets that are figure-8-shaped. When at rest, the wings lie parallel to each other on the abdomen, with the cilia of the posterior margins in the midline, but the wing-holding mechanisms vary considerably between species. The 10th abdominal segment of Terebrantia species is incomplete ventrally, and the ovipositor comprises four sawlike blades that are used to insert an egg into plant tissue.
FAMILY CLASSIFICATION
Species, or groups of species, with particularly unusual structures are sometimes segregated into smaller families in both suborders. Despite this, the suborder Tubulifera is usually considered to include a single family, the Phlaeothripidae, with 3500 species. The Terebrantia are more diverse in structure, and eight families are currently recognized for the 2400 species.
Phlaeothripidae
Two subfamilies of Phlaeothripidae are recognized with about 600 species that feed by ingesting whole fungus spores comprising the Idolothripinae. These include some of the largest thrips with body sizes up to 15 mm. Males are often considerably larger than females, with prominent tubercles and large foretarsal teeth. Moreover, these large thrips commonly exhibit patterns of allometry, such that the largest males differ considerably in structure and appearance from the smallest in the same population, and these size differences reflect fighting behaviors.
The larger subfamily, the Phlaeothripinae, comprises three ill-defined lineages. The Haplothrips lineage, or Tribe Haplothripini, is common in the north-temperate zone in the flowers of Asteraceae and Poaceae, including cereal crops, and is widespread across the Old World tropics. The Liothrips lineage occurs worldwide, particularly in warmer areas, and the species feed on the leaves of shrubs and trees, commonly causing them to distort, to roll, or to form discrete galls. The Phlaeothrips lineage occurs worldwide, and comprises species that feed on fungal hyphae on dead branches or in leaf litter. Many of these exhibit complex allometry in males, form colonies, and are subsocial. Species recognition and generic classification within the Phlaeothripidae is unsatisfactory, because of the lack of studies on biology and structural variation within and between colonies. Isolated individuals can often not be identified.
Thripidae
This is the largest of the eight families of Terebrantia. Adults of all 2000 species have slender, emergent, simple or forked, sensoria located on the third and fourth antennal segments. The greenhouse thrips, Heliothrips haemorroidalis, is placed in the subfamily Panchaetothripinae, together with about 130 related species that have a body and legs strongly reticulate. Similar small numbers of species are placed in two further subfamilies, Dendrothripinae and Sericothripinae, but most thripids are placed in the Thripinae. This subfamily includes most of those flower-living insects that are commonly recognized as thrips, particularly the 450 members of the two genera Thrips and Frankliniella, many of which are important crop pests (Fig. 1D).
Aeolothripidae
The 200 species in this family all have nine-segmented antennae with at least the last three segments closely joined, and the sensoria on the third and fourth segments are almost always linear along these segments. Aeolothripids are relatively large thrips, with the forewings broadly rounded at the apex and commonly banded black and white. Although they are probably mainly predatory on other arthropods, the common flower-living members of the genus Aeolothrips also feed on plant tissue, the only obligate predators being tropical species in the genera Franklinothrips, Mymarothrips, and Stomatothrips.
Melanthripidae
The 75 species in this family are all flower feeders. In contrast to aeolothripids, all nine antennal segments are distinct and bear transverse rows of microtrichia, and the sensoria on the third and fourth segments are linear around the apex. Moreover, on the abdomen, remnants of an eighth sternite are visible, as in Merothripidae.
Heterothripidae
The 70 species recognized in this family are found only in the New World. The adults all have antennae with nine segments, and the sensoria on the third and fourth segments are continuous around the apex. One Brazilian species is ectoparasitic on a species of plant hopper (Fig. 2A), but the other species probably all breed in flowers, some being host-specific.
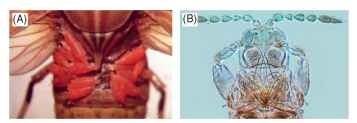
FIGURE 2 Thysanoptera diversity. (A) Aulacothrips dictyotus larvae, ectoparasitic on Aethalion in Brazil. (B) Adrothrips intermedius adult from Casuarina in Australia.
Minor Families
Scarcely 1% of Terebrantia species comprise the remaining four families. A single, widespread, tropical species is placed in the Uzelothripidae. This is presumed to be fungus-feeding and has a remarkable whip-like terminal antennal segment. The Adiheterothripidae includes two species from the western United States and four species from the flowers of date palms between the eastern Mediterranean and India. The Fauriellidae includes one species from California, two from southeastern Africa, and two from southern Europe. The family Merothripidae includes about 20 species, mostly from South America, all of which feed on fungus on dead twigs and leaves. These are minute thrips, but they retain structural character states that are presumed to be closest to the ancestral states of all Thysanoptera.
LIFE HISTORY
Reproduction in thrips is haplodiploid, which involves males developing from unfertilized eggs and having one-half the number of chromosomes of females. Despite this, several thrips species can produce females from unfertilized eggs, a process known as thelytoky. In a few common and widespread species, such as the greenhouse thrips H. haemorroidalis, males are rare or, as in the North American bass-wood thrips, Thrips calcaratus, unknown. Larvae usually hatch within a few days, but in some of the larger Idolothripinae eggs develop while still in the abdomen of a female and larvae hatch soon after the eggs are laid.
There are two larval stages, both of which feed actively for about 2-5 days. In Terebrantia there are then two pupal stages with the antennal segmentation reduced or absent and the mouthparts nonfunctional. Wing rudiments can be seen in the first, the propupa, but are much longer in the second, the pupa. Tubulifera species are even more remarkable in that they have three pupal stages.
Some Thripidae and Phlaeothripidae pupate on leaves in association with their larvae, but more commonly pupae are found in the leaf litter or on the trunks of trees. The second instar larvae of Aeolothripidae, Heterothripidae, and even a few Thripidae spin a pupal cocoon. Larvae in some species have stout tubercles near the posterior end of the abdomen that presumably assist burrowing into the soil to pupate.
Species that are host-specific within the flowers of particular plants are usually univoltine. Related polyphagous species often breed continuously as long as conditions remain suitable. Overwintering stages are usually pupae, or adults, but the citrus thrips, Scirtothrips citri, overwinters as eggs in leaves and shoots.
FEEDING
The feeding stylets of phytophagous, predatory, and fungus-feeding thrips are essentially similar. The two maxillary stylets fit together along their length with a tongue-and-groove system, and their apices are linked with slender finger-like projections around a subapical feeding aperture. Only in the spore-feeding Idolothripinae are the stylets clearly adapted to the food consumed, being exceptionally broad to facilitate the ingestion of whole spores.
The only obvious difference in stylets between species is their length. Among the Terebrantia, the maxillary stylets are short and restricted to the mouth cone, but in many Phlaeothripidae, the stylets are deeply retracted into the head, sometimes as far as the eyes, and lie close together along the midline. In a few species feeding on Casuarina trees in Australia, the stylets are longer than the total body length and are coiled within the head (Fig. 2B).
When feeding, a thrips initially makes a hole by extruding the solid needle-like mandible. This is then withdrawn, and the maxillary stylets are inserted into the food source through the hole, saliva being pumped into the tissues and the resultant fluid pumped back into the thrips’ crop. In those thrips species in which adults transmit tospovirus diseases to plants, the virus is acquired only by a first, or early second, instar. The virus is taken up from an infected cell when a larva feeds. The virus passes through the wall of the fore gut into the salivary glands, from where it is reinjected into a plant by the adult thrips. Although an adult may take a tospovirus into its gut, the virus is not able to reach the salivary glands, so that no adult can acquire and then transmit a tospovirus.
HOST RELATIONSHIPS
The precise host relationships of most thrips species remain unknown. For example, of the 43 endemic North American species of genus Thrips, the larval host is not recorded for any one species. A major reason for this lack of basic biological information lies in the dispersive activity of thrips, adults sometimes being found in very large numbers on plants on which they cannot breed. Despite this, some host-plant relationships are well established. The Palearctic species of Odontothrips and the Old World tropical species of Megalurothrips breed only in flowers of the family Fabaceae. Similarly, Dichromothrips species live on Orchidaceae in the Old World; Projectothrips species live only in the flowers of Pandanus . the screw pines of the Old World tropics; and all four species of the adiheterothripid genus Holarthrothrips live only in the flowers of the date palm, Phoenix dac-tylifera. The Poaceae has a particularly rich fauna of Thripidae, with Aptinothrips and Limothrips being specific to grasses in the Palearctic; Stenchaetothrips, Fulmekiola, and Bregmatothrips specific to grasses in the tropics; and Chirothrips and Arorathrips breeding in grass flowers in many parts of the world.
In contrast to these host associations at genus level, some thrips genera show a different pattern of host exploitation, with each species using as host some unrelated plant. In the New World genus Echinothrips, one species lives on a species of Selaginella (Lycopsida), another lives on the needles of Tsuga (Pinaceae), a third lives on various soft-leaved plants in Euphorbiaceae and Balsaminaceae, and a fourth is polyphagous with no clear host associations. In Australia, most Odontothripiella species breed in the flowers or Fabaceae, but at least one breeds in the flowers of native Poaceae.
Biological data are particularly weak in the Phlaeothripidae, with many species being known only from single samples. In Australia, one suite of about 200 species of Phlaeothripinae is associated only with Acacia, and this suite of species appears to represent a single evolutionary lineage. This contrasts with the complete absence of Phlaeothripinae from the leaves of any of the 900 species of Eucalyptus and also contrasts with the host shifting to unrelated plant families among Australian gall-inducing Leeuwenia species.
Almost nothing is known of host specificity in fungus-feeding thrips. In Europe, certain species of the phlaeothripine genus Hoplothrips are associated with Stereum fungi on dead branches of trees. The tropical species of Idolothripinae that have exceptionally broad stylets presumably feed on larger fungal spores than related species with slightly less broad stylets, but such details of thrips natural history continue to be little studied.
BEHAVIOR
In Terebrantia, males are usually much smaller than females, whereas in Tubulifera males are commonly much larger than females. These differences are related to different patterns of sexual behavior. In fungus-feeding phlaeothripids, a male will defend a female and her egg mass or alternatively will defend a single egg mass to which various females contribute after first mating with him. These strategies lead to competitive behavior between males, involving flicking with the abdomen to displace a rival or stabbing with foretarsal teeth to kill a rival. Moreover, while large males are fighting, small males may sneak-mate. Clearly there is a balance of advantages, between developing quickly on a small amount of food into a sneaky small male and developing slowly on more food into a fighting male.
Competition between males is possibly an ancestral trait in Thysanoptera, because in the basal clades Merothripidae and Aeolothripidae males of some species are polymorphic and presumably competitive. In Merothrips and Cycadothrips species, the largest and smallest males differ considerably in body size and in the strength of their forelegs and abdominal setae. Male competition also occurs in some Thripidae, including the pest species F. occiden-talis. Males of Kelly’s citrus thrips, Pezothrips kellyanus, form leks on ripe lemons, and females are attracted to these male aggregations for mating, and this usually occurs in the late afternoon.
THRIPS DOMICILES
The term “domicile” is used to include leaf galls that are induced by thrips and also the shelters that many Australian Phlaeothripinae construct by fixing leaves together with glue or silk. Gall induction by species of Phlaeothripinae is widespread in tropical countries although inadequately recorded in the Neotropics. Galls range from simple rolled leaves containing a few thrips to highly contorted masses of leaf tissue enclosing up to 10,000 adults and larvae. In most gall-inducing thrips from the Oriental Region, there is little sexual dimorphism, whereas gall thrips on Acacia and Casuarina trees in Australia exhibit considerable sexual dimorphism as well as long- and short-wing morphs.
In some phlaeothripines on Acacia in Australia, the gall foundress is a fully winged female (Fig. 1B), but the eggs she lays first develop into short-winged adults of both sexes (Fig. 1C). These adults sometimes have reduced reproduction and act as soldiers to defend a gall while the foundress produces a second and larger generation that become winged adults. This behavioral strategy falls within the definition of eusociality.
The habit of domicile construction by thrips is recorded only from Australia, in a suite of species on Acacia. At least 30 species are now known to form such shelters using a secretion from the anus. In some of these species, the leaves are glued together in pairs at an angle, and the thrips breed within the space created by a ring of glue and the two surfaces. In other species, the secretion is more silken in form, and this silk is used to sew together two or more leaves enclosing a small space within which the thrips breed. At least two species are known to use this silken material to weave a tent on a leaf surface within which to breed.
The hot dry climate of Australia leads to competition between thrips for the protection afforded by these domiciles. Various klep-toparasitic species have evolved, each with a different way of usurping a domicile. Some drive out the original inhabitants using a frontal assault with sharp foretarsal teeth whereas others use a porcupinelike action of their abdomen that bears many stout setae. A few species have evolved as true inquilines and can breed within a domicile without unduly disturbing the original inhabitants.
FLIGHT AND DISTRIBUTION PATTERNS
In warm humid weather, adult thrips often climb to the tops of plants or dead twigs, from where they are readily dispersed by air currents. Many species then actively jump into the air, and before take off, winged Thripidae comb the marginal cilia of their wings from a parked position parallel to the wing margin into a flight position at right angles to the wing. However, even wingless individuals are dispersed by the wind. Wingless species from the mountains of southeastern Australia can reach the northern part of South Island, New Zealand, a sea crossing of more than 1600 km. Such dispersal is probably not merely fortuitous, but is a function of the behavior of particular thrips species. Some, such as Frankliniella schultzei in Australia, seem to be particularly prone to long-distance migration, whereas the extensive dispersal of the western flower thrips, Frankliniella occiden-talis, is primarily due to the horticultural trade.
The worldwide distributions of many thrips species result from our trading patterns. For example, Chirothrips species pupate within the glumes of grass florets and are widely distributed in commercial grass seed. Other grass thrips were distributed in hay on sailing ships during the period of colonial expansion, and species of the European genera Aptinothrips and Limothrips can now be found in temperate zones all over the world. Similarly, orchids, bananas, and sugarcane, all of which are transported and planted from plant parts, not seeds, have been accompanied around the world by their pest thrips species. The vast increase in the use of air transport by the horticultural trade since 1980 continues to expand the world distributions of pest thrips.
Some distribution patterns are clearly natural. The family Heterothripidae is confined to the New World, and most species of Merothripidae are also restricted to that area. In contrast, the genus Thrips, with 285 species worldwide, has no species native to the Americas south of Mexico, and the genus Frankliniella, with 220 species, includes very few that have a natural distribution anywhere outside the New World. Presumably these two advanced genera of Thripinae evolved at about the time that the American continent separated from Europe.
Within the Melanthripidae, the genus Dorythrips has three species in southern South America and two in Western Australia and Cranothrips has one species in South Africa and several in Australia. Among the more advanced Aeolothripidae, the two genera Aeolothrips and Desmothrips are ecological and morphological counterparts of each other, the first restricted to the Holarctic, the second to Australia.
Geographical distribution patterns are less clear among the Phlaeothripidae. The leaf-feeding members of the large genus Liothrips are found throughout the tropics, including the Pacific region, but species of the closely related genus Gynaikothrips are Asian in origin. The large thrips of the genus Elaphrothrips, all of which feed on fungal spores, are found widely throughout the tropics, but east of Wallace’s Line they are replaced by members of the genus Mecynothrips.
POLLINATION
Thrips are sometimes abundant in flowers, and they can fly actively between flowers. However, their function as pollinators is frequently overlooked; no less than Charles Darwin complained of thrips interfering with his experiments on larger pollinators! Although a single thrips may carry only 10-50 pollen grains, the large number of thrips in each flower can deliver sufficient pollen to stig-matic surfaces. Thrips have been demonstrated to be pollinators in a wide range of flowers: heather plants in the north of the Northern Hemisphere, dipterocarp trees in Malaysia, a rain forest tree in eastern Australia—Wilkiea huegeliana (Monimiaceae), the Panama rubber tree—Castilla elastica (Moraceae), several Macrozamia cycad species in Australia, and Antiaropsis decipiens— the sister group to Ficus (Moraceae) in New Guinea.
PEST SPECIES
In general, it is only pest thrips that are noticed by nonspecialists. Phlaeothripidae species are rarely pests, although in central Europe cereal crops are sometimes damaged by one species, and other species cause leaf damage in the warmer parts of the world on decorative Ficus trees, black pepper vines, and olives. Most pest thrips, and all the tospovirus vectors that cause such serious economic losses, are members of the Thripidae. Financial losses in Californian and South African citrus production can be severe, due to the skins of fruit being scarred by thrips feeding. Similarly, the value of a nectarine can be seriously reduced through a single thrips larva feeding on the fruit when it is young. Cucumbers, capsicums, and strawberries are badly distorted at times because of the feeding activity of thrips. Roses, carnations, and chrysanthemum flowers can all be devalued through thrips feeding damage, and table grapes burst and become fungal-infected through thrips oviposition scars.
Worldwide, there are four species of thripids that are particularly significant as pests: the onion thrips (Thrips tabaci), the melon thrips (Thrips palmi), the tomato thrips (Frankliniella schultzei), and the western flower thrips (F occidentalis). Although feeding damage by these can be severe, the tospoviruses that each of them transmits are a greater problem. More than 12 of these viruses have been described, and although they cause damage only to plants it is evident from their molecular structure that they are members of the animal virus family Bunyaviridae. The origin of the plant infections remains unknown, but each tospovirus is dependent for its continued existence on being transmitted from one plant to another by one or more of 10 thrips species.
