The body temperature of most insects is linked to changes in ambient temperature. Insects are ectothermic and hetero-thermic (poikilothermic) organisms, in contrast to endother-mic and homeothermic birds and mammals. Large dragonflies, hairy bees, and moths generate and maintain a high and stable thoracic temperature during flight.
Temperature fluctuations are small in environments such as the tropical rainforest, caves, and large streams and water bodies. In most terrestrial habitats, the seasonal and diurnal temperature oscillations are considerable. The body temperature of insects can change rapidly by 10°C or more when shade and direct sunlight alternate. Insects heat up by sunlight through basking behavior and thermal melanism.
Preference for living at high temperatures (in warm regions or on a warm-blooded host) is called thermophily, whereas inclination to low temperatures is psychrophily (usually soil and aquatic arthropods). Temperature within the species-specific physiological range determines the rates of metabolism, growth and development, and often exerts other physiological effects.
METABOLIC RATE
The metabolic rate (mr) reflects the velocity of biochemical processes and can be measured as oxygen consumption, carbon dioxide production, or heat generation. Metabolic increase is exponential, usually two- to threefold with temperature increase by 10°C (so-called Q10): mr = ea+kT. Metabolic rate gradually decreases with decreasing temperature even in immobile insects in a cold stupor.
Weight-specific metabolic rate (in ml O2 or CO2g-1h-1) is higher in smaller individuals, and in insects acclimated to lower temperatures.
Active metabolic suppression mediated, for example, by downregula-tion of important enzymes enables survival for long periods in a dormant state without feeding, even at relatively high temperatures. The effect of temperature on metabolism of poikilotherms may explain its effects on growth rate and developmental time.
GROWTH RATE AND BODY SIZE
Three factors control the size that insect larva attains: (1) the growth rate of the last instar larva, which determines how much weight an individual can gain in a given time period, and which is strongly positively temperature dependent; (2) the critical weight, which is the mass at which juvenile hormone secretion stops and which is temperature independent; and (3) the interval to cessation of growth (ICG) after the critical weight is attained (the larva may double its weight during this period) decreases with increasing temperature.
The function that describes the dependence of a phenotype (such as body size) on a particular environmental variable (such as temperature) is called a reaction norm. The relationship between growth rate and temperature is often linear over the range of physiologically suitable temperatures and can be expressed as: gr = aT-b. The ratio b/a is the threshold temperature T0, the hypothetical temperature at which the growth rate would be zero (Fig. 1 ) .
Adult insects generally are of smaller body size when larvae are reared at higher temperatures. For example, females of Bicyclus butterflies reared at 20°C were larger than those reared at 27°C. Moreover, females laid larger eggs when they were reared or acclimatized for 10 days at the lower temperature compared to the higher temperature.
Regulation of body size can be partially explained through mechanisms that control cell size and cell number. However, size regulation also requires mechanisms that take information on a scale related to the regulated organ. In insects, this mechanism controls the secretion of ecdysone, which terminates the growth phase of development. The regulation of the size of the entire body must either respond to information produced all over the body, or it must be sensitive to the size of a particular “indicative” body parts.
Relative proportions of body parts are almost constant with changing absolute body size related to developmental temperature (isometric growth). Insulin signaling pathway affects the growth of body parts in a proportional fashion. Spatial changes in tissue receptor activities may account for the variability of body proportions and allometry (e.g., in males of stag beetles, Lucanidae).
Robertson found that genetic differences in wing size in different strains of Drosophila melanogaster were mainly due to genetic differences in cell number and cell size remaining almost constant. By contrast, in a given strain of Drosophila, the increase in the size of body parts at lower rearing temperatures was consistently due to a change in cell size and not in cell number.
DEVELOPMENT RATE
Development time (dt) is the time required to complete specified stage or instar and can be described as dt = SET/(T-T0). SET is the sum of effective temperatures or “thermal constant,” expressed as the number of degree days. T0 is the lower developmental threshold (LDT, or base temperature Tb), the hypothetical temperature at which developmental time would be infinite or developmental rate would be zero. The product of developmental time and the amount to which ambient temperature is above the threshold was found to be constant (= SET), that is, development will take a fixed number of
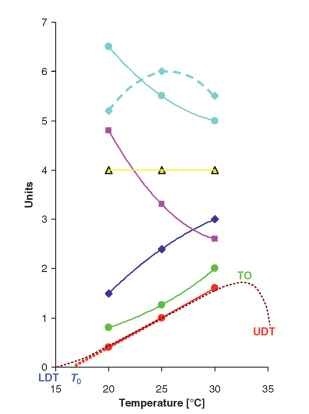
FIGURE 1 Reaction norms of physiological properties of idealized larval instar reared under three constant temperatures: green = metabolic rate (oxygen uptake) (ml g-1h-1)—exponential increase with temperature; blue = growth rate (gday-1)—linear or slightly convex increase with temperature; yellow = critical weight (g) when release of juvenile hormone ceases—i ndependent on temperature; magenta = interval to cessation of growth (days) after critical weight has been achieved—concave decrease with temperature; cyan = peak body mass (g)—either concave decrease with temperature (solid line) or convex trend with optimum (maximum) at moderate temperatures (dashed line)—growth continued for different time intervals after critical mass has been achieved; red = developmental rate (week- 1)—linear or sigmoidal (brown dotted curve) increase with temperature. LDT: actual lower developmental threshold; T0: predicted lower developmental threshold; UDT: upper developmental threshold; TO: thermal optimum (maximum) for developmental rate. Total optimum for population growth is usually at moderate temperatures, not at such high extremes.
degree days essentially independent of the temperature at which the animal is reared. The thermal parameters are determined in defined conditions (set of constant temperatures, suitable nutrition).
For example, a SET value of 100 degree days means that development at 5°C above LDT lasts 20 days and at 10°C above LDT 10 days. When the insect develops at fluctuating temperatures, the average temperature above LDT and the length of time when the temperature surpasses LDT are considered for each day, and the number of degree days established in this way is summed. Developmental stage (instar) is completed when the summation reaches SET value.
A temporal drop in temperature below LDT is associated with developmental block and is counted as zero. Usually, there is no
” negative developmental rate” at temperatures below LDT, that is, no delay of development is observed after transfer to an effective temperature. Natural temperature fluctuations, however, may have a signaling effect and influence the SET value in some species, and this possibility needs to be checked experimentally.
The LDT and SET values are population-specific characteristics. The LDT values are similar for all developmental stages of a given population, even when they develop in diverse seasons and experience disparate temperature fluctuations. The stability of LDT is manifested as developmental rate isometry, that is, the percentage of time spent in a particular stage at any constant physiological temperature is a stable fraction of the entire developmental time.
The LDT and SET values established in the laboratory enable prediction of the course of development in the field. On the basis of daily temperature measurements, we can predict the time of the first egg laying and of larvae hatching, and thus appropriate timing of insecticide spray against the particular pest.
Both LDT and, especially, SET may vary between geographical populations because of adjustments to local climatic conditions. There is a trade-off between LDT and the thermal constant (SET) that enables each species to adapt to its thermal environment. Tropical species have higher values of LDT than temperate ones. SET decreases as LDT increases. Insects that have spread to temperature zones from the tropical regions often maintain a high LDT and can reproduce and develop only in the hot season, spending most of the year in a state of dormancy.
As development rate (dr) is difficult to define and quantify, it is typically approximated by the inverse of development time: dr = 1/dt. Circadian rhythmicity of hatching and molting complicates assessment of dt. In contrast to the exponential rise of the metabolic rate, development rate is roughly linearly dependent on temperature: dr = (T-T))/SET. A possible basis for the linear relationship between rate and temperature is proposed based on the Arrhenius and Sharpe-Schoolfield equations involving activation enthalpy and progressive inactivation of the reactant molecules at both low and high temperatures.
The trend line deviates from linearity at both low temperatures near lower developmental threshold (LDT or T0) and high temperatures near upper developmental threshold (UDT). The relationship between developmental rate and temperature is thus best fit by a logistic equation (S-shaped curve): dr = ea+bT/(1+ ea+bT). Actual LDT is therefore somewhat lower than predicted T0. Developmental rate reaches maximum at the “optimal” temperature near UDT.
DORMANCY AND PHENOTYPIC PLASTICITY
Special set of effects of temperature on development is connected with developmental arrest. The state of easily reversible, temperature-dependent developmental arrest is known as quiescence. It is typical of insects adapted to relatively short periods of unfavorable, nonlethal circumstances. Insects that will be exposed to harsh conditions lasting for many months enter a programmed developmental arrest, diapause. Diapause is induced by environmental signals (typically photoperiod) acting before coming of the adverse conditions. Low temperature may increase incidence of diapause induced by short photoperiod. For example, 50% of caterpillars of Acronycta rumicis are induced to enter pupal diapause at a daylength of 19 h at 15°C, but at 16h at 25°C.
Larvae of the wax moth, Galleria mellonella (Lepidoptera, Pyralidae), normally develop at high constant temperature about 30°C in the beehive. If exposed to suboptimal temperature of 18°C,a developmental arrest occurs as the consequence of low ecdysteroid titer. It was described as facultative larval diapause, although transfer back to the optimal 30°C results in the immediate resumption of development.
Daily fluctuations of temperature, the thermoperiod, can induce diapause in a few species kept in constant darkness. Once induced, diapause requires a set time, typically months, during which neuro-hormonal regulations return to the pattern supporting development and reproduction. In the overwintering insects, diapause is often shortest at temperatures around +5°C. Due to exposure to low temperatures in late fall, the overwintering insect terminates diapause in early winter, and the resumption of its development or reproduction is then halted only by a direct effect of low temperature: diapause turns into quiescence.
Predictable habitats permit fixed life cycles: for example, development in summer and diapause in winter. However, in many species from unpredictable habitats, individuals can follow alternative life-cycle pathways, resulting, for example, in cohort splitting.
Changes in the number of molts have been noted in many insects. Larvae of the mealworm, Tenebrio molitor, develop in 11-15 instars at 25°C and in 15-23 instars at 30°C. Chilling stress (short exposure to 0°C) in Galleria mellonella causes supernumerary moltings induced by persistent increase in the juvenile hormone titer.
Some insect species occur in more than one form and their alternation depends on temperature. Development of caterpillars of Colias eurytheme at 18°C leads to mainly yellow butterflies, whereas development at 27-32°C produces orange adults. Progressively declining temperatures during larval development of the Bicyclus butterflies produce a generation of adults with delayed reproduction, quiescent behavior, and a cryptic wing underside, whereas high temperatures produce active, fast reproducing adults with an aposematic eye pattern. Acclimation to low temperature can cause reversible changes in body coloration-l acewings and pentatomid bugs turn from green to yellow or reddish brown.
EXTREME TEMPERATURES
A general response of insects to temperatures just below their LDT or above their UDT is the cessation of development and reproduction while the insects remain active and feed. The larvae may slowly grow and the adults accumulate reserves. These processes are terminated at more extreme temperatures.
During cooling, motility gradually decreases. At certain temperature, the neural and muscular activities are impaired and the insect lapses into cold stupor (chill coma). The stupor point is as high as 12°C in tropical insects including stored product pests, and in honey bees, around 5°C in many temperate species, near 0°C in most overwintering insects, and even below the freezing point in species living in very cold areas.
Desiccation and nutrient depletion during the cold-induced starvation are certainly incompatible with long-term survival, but death usually occurs earlier and is probably the result of damaging effects at cellular level. The loss of cell membrane fluidity and imperfect protein functions (enzymatic activity, transport, etc.) causes metabolic disorders. The ion pumps in the cell membrane become inefficient and sodium concentration in cytoplasm increases, while the potassium ions flow out into the hemolymph.
Gradual warming above UDT, which is for many species around 35°C but is never sharply delimited, increases the metabolic rate, loss of water, and motility. Around 40°C, the water loss increases sharply: the spiracles are wide open and the melting of cuticular lipids permits evaporation through the body surface. Exhaustion of water and nutrients leads to rapid decrease of motility and a drop of transpiration. At a certain temperature, heat stupor occurs. Survival at temperatures above the threshold is a function of temperature and length of exposure. Warming to the absolute upper lethal temperature, which is usually around 50-55°C, causes fast, irreversible tissue damage and death.
Heat shock proteins are typically expressed during mild, high-temperature exposure, protecting the organism at even higher temperatures. It is believed that they are chaperones enabling or protecting functional protein conformations.
