that could be transmitted to humans. The Food and Agriculture Organization and the World Health Organization (FAO/WHO) broadened the definition during the 1950s to include infections that are transmitted in either direction between humans and other vertebrate animals. Thus, zoonoses are those diseases and infections for which the agents are naturally transmitted between vertebrate animals and humans. Zoonoses can be transmitted to humans by direct contact with an infectious vertebrate host or a fomite (i.e., an inanimate object such as an article of clothing that can harbor an agent); by ingestion of contaminated water, food, or other organic matter; by inhalation; or by arthropod vectors. This topic is devoted exclusively to zoonotic agents that are transmitted from other vertebrates to humans by arthropods. It does not include nonzoonotic disease agents that are transmitted solely from person-to-person by arthropods (e.g., malaria). The terms arthropod vector vs. vector, and arthropod-borne vs. vector-borne, are used interchangeably.
CHARACTERISTICS
Viruses, prions, bacteria (which include the rickettsiae), fungi, protozoa, and helminths can serve as zoonotic agents. Taylor and co-workers reported in 2001 that 868 (61%) of 1415 infectious organisms known to be pathogenic for humans are zoonotic in origin, and that 22% of the zoonotic pathogens can be transmitted by arthropods. An updated literature survey in 2005 similarly identified 1407 human pathogens, 816 (58%) of which are zoonotic. Furthermore, two of the three contemporary internationally quarantinable diseases are caused by vector-borne zoonotic agents that are transmitted either by mosquitoes (yellow fever virus) or fleas (plague bacterium). Plague also is transmitted by other routes, but the bacterial agent (Yersinia pes-tis) is maintained primarily in natural cycles involving rodents and their associated fleas. In the tropics and subtropics, the most important arthropod-borne zoonotic disease agents are transmitted by flies (Diptera) such as mosquitoes, phlebotomine sand flies, and tsetse flies, whereas in temperate regions of the Northern Hemisphere the predominant zoonotic agents are transmitted by ticks.
Humans are “dead-end” hosts for most vector-borne zoonotic agents, that is, secondary cases do not occur following human infection. Notable exceptions are exemplified by yellow fever, plague, and Chagas disease. Humans infected with yellow fever virus can serve as a source of infection for uninfected mosquitoes that feed on them, individuals manifesting primary or secondary pneumonic plague potentially can transmit Y. pestis to other persons by the respiratory route, and people infected with the trypanosome causing Chagas disease can infect triatomine bugs that bite them.
Several factors that affect the ability of zoonotic agents to produce disease in people include the route of transmission (portal of entry), the genetically determined invasiveness (pathogenicity) of the infecting organism, one’s age, and the immune and nutritional status of an exposed individual. Certain of the North American mosquito-borne viral encephalitides may produce more severe disease in disparate age groups. For example, children infected with western equine encepha-lomyelitis virus (WEEV) experience graver illness than adults, whereas the reverse is true for individuals infected with either St. Louis encephalitis virus (SLEV) or West Nile virus (WNV). Zoonoses may cause comparable harm to humans and other animals, or they may adversely affect one group or the other disproportionately. Thus, some mosquito-borne encephalitic viruses (e.g., eastern equine encephalitis virus [EEEV], Venezuelan equine encephalitis virus, WEEV, WNV) can be highly pathogenic for both humans and horses.
Most arthropod-borne zoonotic infections can be characterized by their focal distribution within particular geographic landscapes. A natural focus, also known as a nidus, is an area where the complex interaction of biotic and abiotic environmental factors ensures the temporal persistence of a zoonotic agent. This concept of the natural nidality of disease, referred to as “landscape epidemiology,” was developed into a doctrine by the Russian scientist E. N. Pavlovsky in 1939. Accordingly, foci exist where macro- and microclimatic, vegetational, and soil conditions support populations of arthropod vectors, vertebrate reservoirs, and the agent.
Foci can be as distinct and limited geographically as the burrow of a small mammal or a tick-infested cottage, or they can be diffuse in which case they encompass much broader territories such as coniferous or mixed hardwood forests. Individual cases or localized outbreaks of relapsing fever in California usually are associated with rodent- and tick-infested cabins at higher elevations in mountainous regions. In such foci, the spirochete Borrelia hermsii occasionally is transmitted to sleeping individuals by the bite of the rapid-feeding, nocturnally active, soft-tick vector Ornithodoros hermsi.
In contrast, northern Californian foci of the Lyme disease spi-rochete B. burgdorferi, although present in several major habitat types, seem to be most intense in certain leaf-litter or fir-needle areas within mixed hardwood forests where infection prevalences in host-seeking Ixodes pacificus nymphs sometimes reach 20-40%. In one such forest, human behaviors involving contact with wood, such as sitting on logs or against tree trunks, were found to elevate the risk of encountering I. pacificus nymphs vs. activities entailing exposure solely to leaf litter. The tiny nymph, which is about the size of a poppy seed (1.0-1.2 mm long), readily attaches to people but often remains undetected while feeding for up to several days. If a B. burgdorferi-infected nymph is not discovered and removed within a day or two of attachment, an individual may develop an erythematous rash of protean shape within 3-32 days. This rash, known as erythema migrans (Fig. 1) , is pathognomonic for early-stage Lyme disease and commonly is accompanied by nonspecific, flu-like symptoms.
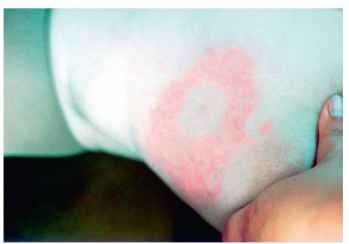
FIGURE 1 Erythema migrans, the skin lesion that is often present during early-stage Lyme disease. It typically occurs at the site of attachment of an infective tick, but multiple lesions may appear on the bodies of some patients.
VECTORS
The phylum Arthropoda contains six classes of varying medical importance but only two of them, the Insecta and Arachnida, are of paramount importance as transmitters of zoonotic agents. Ticks and other mites, solpugids, scorpions, and spiders comprise arachnids of public health interest, but only some ticks and comparatively few mites transmit zoonotic pathogens to humans.
Insects that transmit zoonotic agents belong principally to three orders, namely, the Siphonaptera (fleas), Hemiptera (true bugs), and especially the Diptera (flies). Important families of flies that transmit zoonotic agents are the Culicidae (mosquitoes), Glossinidae (tsetse flies), Psychodidae (phlebotomine sand flies), Ceratopogonidae (biting midges), and Tabanidae (deer flies and horse flies). Other dipteran families, like those comprising the filth flies generally and the house fly in particular (Muscidae), may mechanically contaminate human foodstuffs with bacteria, protozoans, or other agents that can cause gastrointestinal illnesses.
Among the arachnids, ticks overwhelmingly outweigh the mites in importance as vectors of zoonotic agents because of their universal blood-sucking habit. With rare exceptions, all three trophic stages (larva, nymph, adult) of ticks must ingest a blood meal to complete development or to ensure reproductive success. By comparison, most mites are free-living and nonparasitic. In the continental United States, 6 (7%) of the approximately 86 autochthonous tick species are of broad, regional importance because they transmit one or more viruses, bacteria, or protozoan parasites to people with some regularity. Other ticks, like the brown dog tick (Rhipicephalus sanguineus), may serve as primary vectors of disease agents in more restricted geographical areas. This tick recently was implicated for the first time as a vector of the agent of Rocky Mountain spotted fever (RMSF) in the United States, but only in eastern Arizona. One commonality shared by many arachnid and insectan vectors is that they feed intermittently upon their hosts and therefore spend more than about 95% of their entire life cycle off the host.
TRANSMISSION CYCLES
Arthropod-borne zoonotic agents are maintained in transmission cycles of variable complexity. Nevertheless, four components are evident in all such cycles: the agent itself, one or more efficient arthropod vectors and primary reservoir hosts, and a permissive environment. A reservoir host is a vertebrate that is readily infected with the agent, usually is capable of maintaining the agent within its tissues for an extended period, and can serve as a source of infection for uninfected vectors that feed on it while it is in an infectious state. In reality, arthropods also contribute to the maintenance of zoonotic agents to various degrees; some, like certain hard-tick vectors of spotted-fever group rickettsiae or mosquito vectors of some viruses, may be considered reservoirs as well. That is, they can maintain the agent in their tissues for months or even years, efficiently pass it from one trophic stage to the next (transstadial transmission), and eventually transmit it from one generation to the next via the eggs of infected females (transovarial transmission). In such cases, the reservoir of infection may best be considered polyhostal because both the vertebrate host(s) and the vector(s) help to maintain the cycle of infection.
Arthropods may transmit zoonotic agents from an infected vertebrate to a susceptible one either mechanically or biologically. Of these, biological transmission is much more prevalent than mechanical transmission among most groups of arthropod vectors. Mechanical transmission occurs when an agent adheres externally on the mouth-parts, legs, or other bodily regions of a vector and then is transported directly or indirectly to a vertebrate, for example, by means of contaminated foodstuffs or by specific inoculation into the skin or bloodstream by the bite of contaminated mouthparts. In mechanical transmission, the agent does not require the arthropod to complete its life cycle, and the arthropod/agent relationship is accidental in nature.
In biological transmission, the vector plays an indispensable role in the life cycle of the agent. Biological transmission takes three forms: cyclodevelopmental, cyclopropagative, and propagative. In cyclo-developmental transmission, the agent undergoes cyclical changes within the internal tissues of the vector but does not multiply (e.g., mosquito transmission of the filariid nematode Wuchereria bancrofti). In cyclopropagative transmission, the agent undergoes cyclical development and multiplication in the arthropod’s body (e.g., zoonotic babesial piroplasms in their tick vectors or nonzoonotic malarial plas-modia in anopheline mosquitoes). In propagative transmission, the agent multiplies, but undergoes no cyclical development, within the vector’s body (e.g., most bacteria and viruses).
Regardless of the specific mode of biological transmission, the vector usually transmits the agent anteriorally via its salivary secretions while ingesting a blood meal from a vertebrate host. In a few instances, zoonotic agents are transmitted posteriorly when infectious feces deposited on the host are rubbed into the bite-wound, scarified skin, or conjunctivae of the eyes (e.g., feces of triatomine bugs infected with Trypanosoma cruzi, the causative agent of Chagas disease).
CLASSIFICATION
Zoonoses were classified by the FAO/WHO in 1967 according to whether their reservoir hosts are lower animals (anthropozoonoses), humans (zooanthroponoses), or both (amphixenoses), and with regard to the type of life cycle of the infecting organism. The latter classification, which is based upon shared epidemiologic features, is more instructive than one based solely on reservoir hosts. In it, zoonoses have been categorized as direct zoonoses, cyclozoonoses, metazoon-oses, or saprozoonoses. However, only two of these categories involve transmission by arthropods. Direct zoonoses are transmitted, in part, from an infected to a susceptible vertebrate host mechanically by a vector, whereas metazoonoses are transmitted biologically by vectors.
PUBLIC HEALTH IMPORTANCE
The public health and economic impact of the arthropod-borne zoonoses is immense, particularly in developing countries in tropical or subtropical regions. Losses include morbidity and mortality among humans and domestic livestock and the resultant direct and indirect economic effects to affected individuals and to society at large. For instance, the African trypanosomiases still are among the most devastating diseases afflicting humans and livestock. African sleeping sickness is estimated to have killed over 750,000 people between 1896 and 1906, and one epidemic near Lake Victoria in Uganda claimed about 200,000 lives. Recently, it was estimated that over 25,000 new cases of the disease are contracted annually and that 50 million people in 38 countries of sub-Saharan Africa and an even greater number of livestock are at risk. Besides African trypano-somiasis, other zoonotic and nonzoonotic vector-borne diseases such as malaria, dengue, yellow fever, filariasis, leishmaniasis, plague, and louse-borne typhus caused more human morbidity and mortality than all other causes from the 17th to the early 20th centuries, especially in the tropics. Since the 1970s, there has been a resurgence of many of these diseases and the emergence of others (see below), notably tick-borne diseases like Lyme disease, human granulocytic anaplasmosis, human monocytic ehrlichiosis, and human babesiosis in temperate regions of the Northern Hemisphere.
Certain population groups traditionally have been at elevated risk of acquiring vector-borne zoonotic infections. Agricultural and forestry workers, hunters, field naturalists, park rangers, wildlife biologists, woodcutters, and ecotourists represent just a few of the many groups that are vulnerable to such infections. In the northeastern United States, suburbanites may be at considerable risk for contracting Lyme disease in the peridomestic environment because of the presence of host-seeking, spirochete-laden I. scapularis ticks on lawns and in adjacent shrubbery or forested areas. Similarly, in the western United States, the risk of human plague increased in peridomestic settings during the late 20th century as human populations encroached into formerly unpopulated foci of Y. pestis.
EMERGING DISEASES
According to the United States Centers for Disease Control and Prevention (CDC), emerging diseases are those of infectious etiology whose incidence in humans has either increased during the past 20 years or are threatening to increase in the near future. As of 2005, 177 (12.6%) of the 1407 infectious organisms reportedly pathogenic for humans were considered to be emerging. In two published literature surveys (2000, 2005), a higher risk of emergence was found to be associated with zoonotic pathogens (73% or 75% of the emerging pathogens were zoonotic), with certain taxonomic divisions of pathogens (viruses and protozoa were overrepresented), and with vector-borne transmission. Although diverse factors have contributed to the emergence or reemergence of many arthropod-borne diseases during the past 30 years (e.g., agricultural practices, deforestation or reforestation, urbanization), they typically result from environmental changes that produce an increased abundance of arthropod vectors.
In the northeastern United States, human babesiosis and Lyme disease emerged during the 1970s after reforestation and suburbanization combined to increase the abundance of wildlife (e.g., white-footed mice, white-tailed deer) and black-legged ticks (I. scapularis), and consequently the frequency of human-tick contact in the perido-mestic environment. WNV “emerged” in New York State in 1999 following its introduction from the Old World. By 2002, it had achieved pandemic proportions as it swept southward and westward across the country; 4156 cases (284 fatalities) were reported that year by 39 states and the District of Columbia. Additionally, RMSF has reemerged as a major vector-borne disease in the United States as the number of reported cases almost quadrupled between 2000 (495 cases) and 2005 (1936 cases). Indeed, among all cases of indigenous vector-borne diseases reported to the CDC in 2005, the number recorded for RMSF was exceeded only by those for Lyme disease (23,305 cases) and West Nile encephalitis/meningitis (3000 cases).
In Europe, the observed pattern of emergence of vector-borne zoonotic infections parallels the current situation in the United States. Fifteen zoonotic and vector-borne agents were identified as emerging public health threats between 2000 and August 2006, and arthropods are involved in the transmission cycles of two-thirds of them.
ECONOMIC IMPACT
Apart from the human misery and loss of life attributable to zoonoses, the significant costs associated with the occurrence of individual cases or outbreaks of specific arthropod-borne diseases exemplify their economic impact. Among mosquito-borne viruses, an outbreak of SLEV in Dallas, TX, was estimated to have cost nearly $800,000 in terms of morbidity, mortality, patient treatment, and control activities in 1966. In total, 172 presumptive or serologi-cally confirmed cases with 20 fatalities were reported. In New South Wales, Australia, an epidemic of polyarthritis (n = 1196 laboratory-confirmed cases) caused by Ross River virus cost an estimated $3 million in 1983 and 1984. In Massachusetts, the average total cost per case of EEEV was determined to be $21,000 for a transient case vs. a lifetime cost of nearly $3 million for persons suffering severe residual sequelae. In North Carolina, the total estimated medical costs associated with La Crosse encephalitis virus for patients with frank encephalitis averaged $32,974 per patient from onset of illness to date of interview (mean, 3.7 years), and the projected lifetime cost of a case with permanent neurologic sequelae ranged from $ 48,775 to $3,090,798. Lastly, the estimated cost of an epidemic of WNV in
Louisiana (n = 329 cases) from June 2002 to February 2003 was $20.1 million. When these data were extrapolated to all WNV cases (n = 4156) reported nationwide in 2002, the estimated economic impact was $139.8 million even though mosquito abatement and prevention costs were not included.
The economic and societal impacts of flea- and tick-borne zoonotic pathogens can be equally devastating. Following a single human plague case at Plumas-Eureka State Park in Plumas County, CA, in 1976, a benefit-cost analysis of bubonic plague surveillance and control at that park and another nearby campground revealed that the costs incurred when human plague was contracted at either recreational area averaged $52,000. In 2002, when 23,763 cases of Lyme disease were reported to the CDC, the nationwide economic impact was estimated to have been $203 million. This estimate was based on cost data (direct and indirect medical costs, nonmedical costs, and productivity losses) extrapolated from patients seen in five counties in Maryland from 1997 to 2000.
CONCLUSIONS
Zoonoses comprise nearly three-fifths of the 1407 infectious organisms known to cause disease in humans. Among the zoonotic pathogens, —22% are transmitted by arthropods, and many arthropod-borne diseases have either emerged or reemerged since the 1970s. Various factors have contributed to the emergence or resurgence of vector-borne zoonotic pathogens, but foremost among them are ecological changes that give rise to increased densities of arthropod vectors. The economic and societal impacts of certain vector-borne diseases can be staggering, with estimated annual costs averaging up to several thousand dollars per patient. Knowledge of the specific environmental factors, arthropod vectors, and vertebrate hosts contributing to the maintenance of zoonotic agents is a sine qua non for developing and implementing effective preventive or control strategies. This kind of information is vitally important because few vaccines currently are available globally for personal protection against vector-borne infections.
