Intermembrane Sphingolipid Transport
Sphingolipids are unevenly distributed in different subcellular membranes and, as described above, in the single membrane as well. The heterogeneous distribution of complex sphingolipids in the different subcellular membranes, varying from 3-4% of total membrane lipids in the ER to about 20 mol % in the PM and lysosomes,84 strongly reflects the main localization of these lipids in the PM, the topology of the enzymes involved in sphingolipid metabolism and the mode of sphingolipid transport between the different subcellular organelles. Sphingolipid metabolism is indeed compartmentalized, their synthesis starting in the ER and continuing in the Golgi apparatus; degradation mainly occurs in the endosomal/lysosomal district.
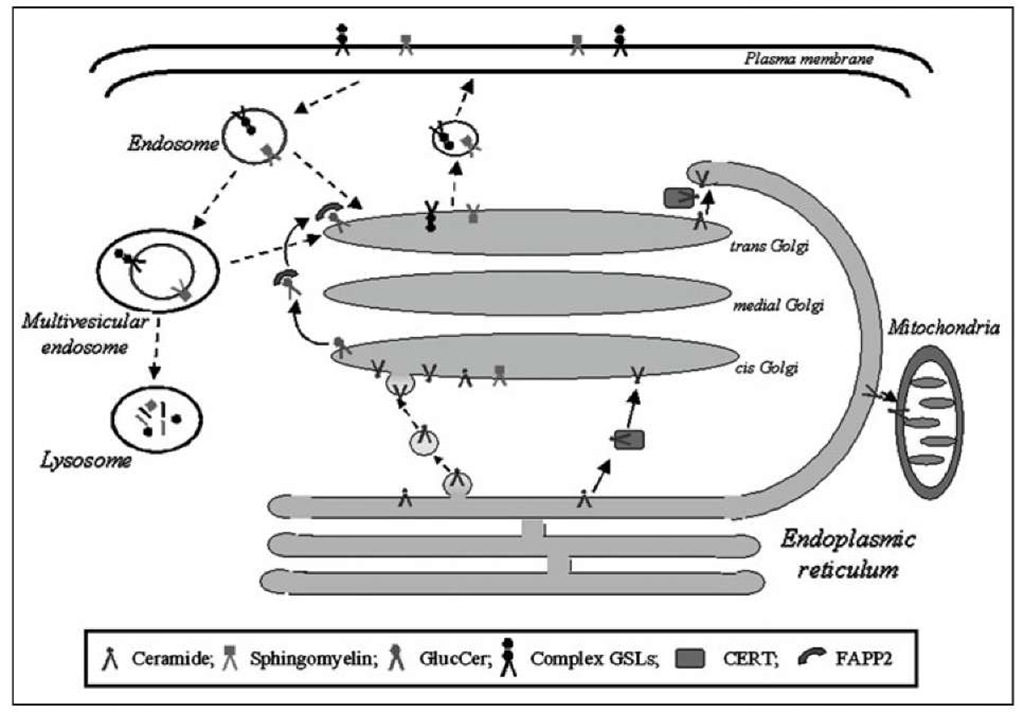
Figure 2. Mechanisms of intermembrane sphingolipid transport. Sphingolipids can move between different cellular membranes via protein-mediated (solid lines) and vesicle-mediated (dotted lines) trafficking routes.
Due to their hydrophobic tail, sphingolipids are not free to diffuse in the aqueous cytosolic milieu. For this reason they must be transported to the different sites of synthesis and degradation.
Two major mechanisms of intermembrane (i.e., between different cell membranes) sphingolipid transport are operative in cells and include protein- and vesicular-mediated transports (Fig. 2).
Protein-Mediated Sphingolipid Transport
In spite the existence of proteins able to transfer sphingolipids in a nonvesicular manner has been postulated more than 20 years ago, in recent years the crucial role oftwo proteins in the transport of selective sphingolipids has been demonstrated. Indeed CERT and FAPP2 (four-phosphate-adaptor protein 2) have been identified as lipid transfer proteins, able to transfer Cer and GlcCer, respectively, to proper membranes in a nonvesicular manner (Fig. 2).
CERT-Mediated Transport of Ceramides
Cer synthesized at the cytosolic face of ER must be transported to the Golgi apparatus to be further metabolized to GlcCer, SM and Cer1P. Before the discovery of the specific Cer transfer protein CERT,85 the studies addressed to understand the mode of Cer transport between ER and Golgi suggested that both vesicular and nonvesicular (possibly protein-mediated) mechanisms can contribute to this process.84
Hanada and coworkers85 identified CERT in a mutant cell line (LY-A cells) characterized by a decrease of SM due to a defect in the transport of Cer to the Golgi.86,87 This finding was pivotal to understand the mechanism of the nonvesicular delivery of Cer to the Golgi apparatus. Studies of Hanada’s group demonstrated that in Chinese hamster ovary cells the biosynthesis of SM but not that of GlcCer is dependent on CERT.85 This was confirmed in different cell lines by different studies88-90 and was mainly attributed to the different localization of GCS and SMS in the cis and trans Golgi respectively. Moreover, down-regulation ofCERT by RNAi strongly reduced the levels of C1P synthesized by Cer kinase (CerK) in the trans-Golgi.91 However, this was not confirmed in other cell types by the use of pharmacological inhibition of CERT,92 this discrepancy being mainly ascribed to the different experimental approach used to inhibit CERT and to the possible different subcellular compartmentalization of CERK.92
CERT is a 69 kDa protein identical to the splicing variant of the Goodpasture antigen binding protein (GPBP) that lacks a serine-rich domain of 26 amino acids. GPBP was previously identified as a Ser/Thr kinase which phosphorylates the noncollagenous-1 domain of the a3 chain of type IV collagen.93 Both the splicing variants can act as Cer-transfer proteins in vitro.85 GPBP and CERT, coded by the COL4A3BP gene, are both highly conserved in the evolution94 and widely expressed in various tissues,95 thus suggesting a differentiated function for the two isoforms. The distinct functions of the two splicing variants GPBP and CERT are also supported by the evidence that they are differentially expressed during Zebrafish development96 and they distinctly concur to the program of protein secretion and Cer traffic in the cell.
CERT has in its structure a pleckstrin homology (PH) domain in the N-terminal region, a START domain in the C-terminal region, a FFAT motif and a serine repeat (SR) motif localized respectively at the C-terminal and N-terminal of in the middle region (MR) between the PH and START domains. All these regions specifically contribute the ER to Golgi Cer transport and its regulation. The START domain is responsible for the Cer binding activity of CERT.85 It shows high structural similarities with START domains of other lipid binding proteins, such as PC transfer protein, the Chol binding protein MLN64 and STARD4, all containing a similarly structured hydrophobic pocket for lipid binding.98 Notwithstanding these similarities, CERT specifically recognizes Cer but not other sphingolipids, phospholipids or Chol and recognizes only weakly diacylglycerol.85 X-ray analysis of the crystal structures of CERT-START domain in its apo- form, or cocrystalized with Cer or diacylglycerol, allowed to identify the structural elements by which CERT can distinguish Cer from other lipids.99 Among the different molecular species of Cer, CERT efficiently transfers those containing C14-C20 fatty acids, but is less efficient towards Cer containing longer chain fatty acids, with the exception of C24:1 species.100,101 This suggests that the major determinant for CERT selectivity is the match between the depth of its hydrophobic pocket and the actual length of Cer acyl chains. CERT can also extract dihydroceramide, but with a relatively restricted fatty acid specificity.
The PH domain restricts the direction of transfer and destination of Cer through its specific binding to phosphatidylinositol 4-monophosphate (PI4P), a phosphoinositide mainly associated to the Golgi. This domain is essential for the correct transport of Cer for SM biosynthesis, since a mutation in this domain is responsible for the defect in Cer transport in LY-A cells.85 The idea that CERT is recruited to the Golgi through the binding of the PH domain to PI4P and this is required for the efficient utilization of Cer for SM biosynthesis is enforced by evidence demonstrating the down regulation or inhibition of PI4 kinase III|3 as a limiting factor for Cer transport and SM biosynthesis in COS-7 cells.89 The FFAT motif is responsible for CERT binding to the ER-resident proteins, vesicle-associated membrane proteins (VAMP)-associated proteins (VAPs). A mutation in the FFAT motif impairs the ER-Golgi transport of Cer, thus demonstrating the crucial role of VAP-CERT interaction in this process.102 These findings indicate that the START domain alone is not sufficient for the transport activity of CERT in vivo and the PH domain and FFAT motif spatially restrict the random Cer transfer activity ofthe START domain to efficiently direct Cer in the biosynthesis of SM. The molecular mechanism of CERT-mediated Cer transport is yet to be defined. At present two main hypotheses can be made (Fig. 3). The first implies a short distance shuttle activity of CERT (Fig. 3A). In this case in its apo form CERT, possibly in a conformation in which the PH domain is masked, binds to ER through its FFAT motif to extract Cer. Cer binding promotes the detachment of Cer-CERT from the ER, the unmasking of PH domain and the docking to PI4P in the Golgi with the subsequent release of Cer. The second model (Fig. 3B) implies that CERT operates in specific regions where ER and trans Golgi come into close apposition, also called membrane contact sites.103 In this model the active form of CERT is simultaneously bound to both VAP in the ER and PI4P in the Golgi.
Figure 3. Proposed models of CERT-mediated transport of Cer between ER and Golgi. A) Hypothetical short distance shuttle activity of CERT; B) CERT may act as Cer transporter at ER-Golgi membrane contacts.
In both cases an open question exists about the signal/s and mechanisms responsible for the molecular dynamics of the process. A crucial point in this context is the phosphorylation state of CERT. In mammalian cells, CERT can be in a hyperphosphorylated state, that involve 7-9 Ser/Thr residues in the SR motif, close to the PH domain.104 This represents an inactive state of CERT, as the treatment of human CERT with a bacterial protein phosphatase strongly increases its activity.104 At present, two protein kinases are known to phosphorylate CERT: the casein kinase I105 and the Golgi-associated protein kinase D.106 In phosphorylated CERT, both the START and the PH domain binding capacity is inhibited, thus suggesting that phosphorylation induces a conformational change bringing the two domains to mutually interfere with each other. Recently it was demonstrated that an ER-resident protein phosphatase 2Ce (PP2Ce) dephosphorylates CERT in a VAP-dependent manner.107 Over-expression of PP2Ce also increase the association of CERT to the Golgi, thus confirming dephospho-CERT as the active transfer protein. The control of CERT phosphorylation by the concerted, but compartmentalized, activities of both protein kinases and phosphatases seems to represent the main element in regulating CERT activity.
CERT activity in SM biosynthesis can be also regulated by the oxysterol binding protein OSBP108 and by the SM/Chol ratio in the PM trough the stimulation of CERT dephospho-rylation,107 thus indicating a role for CERT in the maintenance of SM/Chol homeostasis in the PM.
Different lines of evidence indicate that CERT can be involved in the control of cellular functions other than sphingolipid metabolism. Down regulation of CERT increase the sensitivity to oxidative stress in Drosophila, determining a reduction in fly lifespan.109 CERT is essential for mouse development and embryonic survival, since embryonic death associated to mitochondrial degeneration was observed in CERT-null mouse embryos.110 CERT is up-regulated in drug-resistant cancer cells and down regulation of CERT sensitizes cancer cells to multiple cytotoxic agents.111 Notwithstanding all these effects have been related to the Cer transfer capacity of CERT, the mechanisms involved are still not really clarified and future studies will be addressed to define the role of CERT in the control of cell fate.
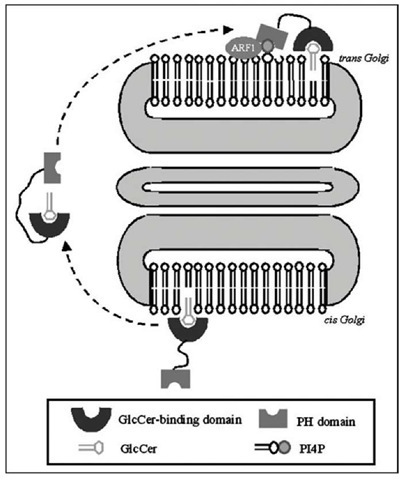
Figure 4. Proposed role of FAPP2 in the transport of GlcCer. According to the actual view, FAPP2 mediates GlcCer transport between cis- and trans-Golgi cisternae.
FAPP2-Mediated Transport of Glucosylceramide
It has been recently demonstrated that FAPP2 mediates a nonvesicular transport GlcCer from cis-Golgi (its site of synthesis) to the trans-Golgi compartments,90 where the enzymes of more complex GSLs the synthesis are localized (Fig. 4). Like CERT, FAPP2 contains a PH domain, PI4P binding to this domain being required for FAPP2 localization to Golgi. Besides this mechanism, the small GTPase ARF1 is also involved in the recruitment of FAPP2 to the trans Golgi network (Fig. 4). FAPP2 also possesses a glycolipid-transfer protein homology domain that is highly specifc for GlcCer, but does not transfer SM, PC or Cer.90 All these observations suggest that GlcCer is transported from the cis side of Golgi to the trans side by FAPP2 in a nonvesicular manner. On the other hand, van Meer’s group presented a different hypothesis64 for this transport: GlcCer synthesized at the Golgi should be retrogradely transported by FAPP2 to the ER, here translocated to the lumen and then transported to the Golgi again to be further glycosylated. So far, whether and how FAPP2 can interact with the ER remains unknown.
Glycolipid Transfer Proteins
Glycolipid transfer proteins (GLTPs) are small, soluble and ubiquitous proteins able to accelerate the intermembrane transfer of glycolipids in vitro.112 GLTP specificity encompasses both sphingoid- and glycerol-based glycolipids, but with a strict requirement that the initial sugar residue be P-linked to the hydrophobic lipid backbone.113,114 Even if early studies with porcine and bovine GLTP established the ability of the protein to selectively accelerate the intermembrane transfer of GSLs in vitro, the mechanism of protein action remained poorly defined.112
Recently, Mattjus proposed that GLTP is not likely involved in the de novo synthesis ofGSLs, but could rather play a role as a glycolipid sensor for the cellular levels of Glc Cer.115 Further studies are required to clarify GLTPs role in sphingolipid transport.
Membrane Contacts
Several organelles make close contacts with each other at zones of apposition called membrane contact sites, which might provide an alternative means of sphingolipid transport. For example, Cer synthesized in the ER has been proposed to diffuse to mitochondria at contact sites (Fig. 2).116 This could be of relevance, as mitochondrial Cer has been implicated in the regulation of apoptosis and no pathway of vesicular transport to mitochondria is known. However, up to know, the transport of different sphingolipids to mitochondria (if any) remains largely unknown.
Sphingolipid Vesicular Transport
Vesicular transport represents a crucial mechanism of sphingolipid intracellular traffic. By this transport different sphingolipids travel as membrane components of small carrier vesicles from a donor compartment to a specific target membrane, where the vesicle fuses. The vesicular transport of sphingolipids is believed to be operated by molecular machineries similar to those of proteins, cytoskeleton proteins and associated molecular motors playing an important role.
Sphingolipid vesicular flow is divided into two major routes depending on its direction in relation to the cell membrane: the biosynthetic and the endocytic pathways. Notably these routes may be interconnected at different levels.
Biosynthetic Vesicular Pathway
The biosynthetic route starts on membranes of the ER where the enzymes for Cer biosynthesis are localised and implies Cer transport to Golgi, where the biosynthesis of SM and GlcCer occurs. Several pieces of evidence support that, besides the CERT-mediated transport (see above), neosyn-thesized Cer at the ER appear to move to Golgi, through a vesicle-mediated route (Fig. 2).85,118,119 Indeed, protein- and vesicular-dependent routes have been shown to coexist in mammalian cells and yeast120,121 and to separately contribute to the control of Cer metabolism and levels.
Vesicular transport appears crucial for Cer delivery to GlcCer synthase. In fact, CERT down-regulation inhibits SM biosynthesis, but not that of GlcCer in different cells.85,88,90 Although ER-Cer sorting into vesicles appears dictated by the specificity of CERT for specific SMs and functional to the transport of Cer for the biosynthesis of GlcCer, the biosynthesis of SM is, at least in part, dependent on the vesicular transport of a pool of Cer to SM synthase.88,120
The property of Cer of promoting membrane vesiculation and budding appears involved in the ER-Golgi vesicular transport of Cer.35 In agreement, ongoing Cer synthesis is required for the efficient transport of GPI-anchored proteins from the ER,122,123 suggesting that Cer has a function in the sorting of specific, ER-derived vesicles. Intriguingly, PI3K/Akt has been shown to regulate the ER-Golgi vesicular transport of Cer and consequently Cer levels in C6 glioma cells,124 implying this traffic in Cer-mediated signaling too.
The transport of intermediate and final products of sphingolipid biosynthesis through the Golgi apparatus to the cell surface requires vesicular transport (Fig. 2). In fact, complex sphingolipids are synthesized on the luminal leaflet of the Golgi and in order to reach the PM, they must be sorted from the Golgi into anterograde-moving vesicles.101,119,125 Even though the mechanisms underlying sphingolipid sorting remain unknown, sphingolipid and Chol segregation from coatomer protein I (COPI) vesicles has been shown to occur at the Golgi apparatus.
In polarised cells (such as epithelial cells and hepatocytes) basolateral and apical membranes maintain a different sphingolipid composition, GSLs being enriched at the apical membrane, whereas the basolateral one displays a normal content.119 These differences imply the sorting of distinct molecules and a specialised sphingolipid trafficking en route to the PM. Whereas baso-lateral sorting relies on the recognition of specific targeting proteins by adaptor-protein coats,127 apical protein sorting is stimulated by PKA and appears dependent on the interactions between GSLs and specific glycoproteins (as glycosylphosphatidylinositol (GPI)-anchored proteins) in the trans-Golgi.128 The selective sorting of GSLs in the apical transport remains poorly understood, but it must involve GSL lateral enrichment in budding apical precursor vesicles,125,129 their hydrogen bonding tendency might possibly favour their segregation from other lipids. In hepatocytes, the displacement of type II PKA from its anchoring in the Golgi selectively excludes newly synthesized sphingolipid analogues from the direct Golgi-to-apical surface transport and results in their vesicular transport to the basolateral surface.130 Moreover, GlcCer synthesis inhibition delays Golgi-to-apical surface trafficking of a specific set of protein,130 suggesting that GSLs at the Golgi are sorted into specific vesicles, which are stimulated by type II-PKA and crucial to the delivery of specific proteins to the apical surface.
Endocytic Pathway
In the endocytic pathway, parts of the PM containing sphingolipids bud and are incorporated into early endosomes (Fig. 2). Endocytosis can occur through clathrin-dependent, caveolae-depen-dent or clathrin/caveolae-independent mechanisms, either constitutively or ligand-stimulated.131 In a single cell type multiple endocytic mechanisms with different cargos and protein machinery, may exist.
In early endosomes different and specific steps of sorting gradually occur. This sorting results in the formation of intraluminar vesicles and gives origin to multivesicular endosomes (MVEs or late endosomes). Different components of the Endosomal Sorting Complex Required for Transport (ESCRT) play a role in the formation of intralumenal vesicles, although their precise role remains to be clarified.
It is believed that endocytosis occurs at sphingolipid-enriched regions of the PM and recent studies indicate that variations of specific sphingolipids influence endocytototic flow. For example, addition (or depletion) of GSLs, but not SM, selectively stimulates (or/inhibits) caveolar endocytosis in different cells.133,134 In addition, SM accumulation at the PM, impairs the membrane targeting and activation of Rho A and leads to defects on RhoA-regulated endocytosis.134,135 Thus sphingolipids appears of relevance and differentially required in the regulation of different endocytic mechanisms.
Sphingolipids can be delivered to different membranes as components of both the endo-somal- or of intralumenal-vesicle membranes. After internalization via endocytosis, different vesicles transport sphingolipids to three major destinations, including the lysosomes (degradation pathway), the Golgi apparatus where additional glycosylations of GSLs may take place, or the PM (direct recycling and transcytosis).
The current view is that sphingolipids destined for degradation reach the lysosomes as membrane components of MVE internal vesicle (Fig. 2). After fusion with lysosomes, these vesicles become intralysosomal vesicles, their membranes (internal membranes) providing the platform of sphingolipid (and other membrane components) degradation.136,137
During the maturation of MVEs destined to lysosomes, lipid-sorting occurs and leads to the formation of internal vesicles enriched in bis(monoacylglycero)phosphate (BMP) (incorrectly also called lysobisphosphatidic) and depleted of Chol.137 BMP-containing membranes play a role in GSL degradation136 and regulate the export of Chol from internal membranes138 and lipid sorting/ export from late endosome intralumenal membranes to other cellular destinations.132
Chol removal from internal membrane vesicles is crucial for degradation processes. Schulze et al137 proposed that during endocytosis the decrease in luminal pH is paralleled by SM degradation to Cer by acidic SMase and increased Cer may displace Chol from sphingolipid-enriched domains,139 facilitating its transport out of the lysosomes.
Sphingolipid digestion by lysosomal hydrolases produces several molecules, such as fatty acids, sphingoid bases and monosaccharides, which are not further degraded in the lysosome, but exported and mainly recycled for biosynthetic purposes. These salvage pathways represent an important saving of energy in the cell economy and constitute a relevant event in sphingolipid turnover.140 Despite Sph is efficiently recycled in different cells, the mechanism of its egress from lysosomes is unknown. Since Sph would be expected to partition in lysosomes by virtue of its ionizable positive charge, its exit from the lysosome should be protein-facilitated.
The direct recycling of endocytosed vesicles back to the PM can occur via early endosomes or MVEs. In early endosomes, some sphingolipids together with other lipids and proteins that need to be recycled back to the cell surface, such as receptors, are collected into tubular domains that eventually detach and form recycling endosomes.141 The direct recycling of both fluorescent and natural SMs has been reported to be different, short-chain SMs being more efficiently recycled than the long chain SMs.142 Furthermore, Chol loading or depletion of proteins involved in MVE formation or late endosomal trafficking altered the recycling and/or lysosomal degradation of SMs in a chain length-dependent manner.142 Thus sphingolipid recycling appears a protein- and Chol-regulated process and might involve the specific sorting of sphingolipid molecular species.
At least in some cell types, recycling of MVEs can occur. Upon fusion with the PM, MVEs extracellularly release their intralumenal vesicles as exosomes, which play key role in multiple processes as intercellular communication and antigen presentation.In a recent study performed in an oligodendrocyte cell line, Trajkovic et al34 reported that exosome formation is ESCRT-independent but requires neutral SMase 2-mediated Cer formation on the cytosolic side of endosomes and purified exosomes were found Cer-enriched. The Authors suggested that Cer-induced aggregation of lipid microdomains leads to domain induced inward budding of intraluminal vesicles.
Besides the direct route from the Golgi, polarised cells can employ an indirect route (transcyto-sis) to transport GSLs (and specific proteins) to the apical membrane, involving prior sphingolipid delivery to the basolateral surface followed by rerouting to the apical membrane. For example, in polarized Madin-Darby-canine-kidney cells, newly synthesized GSLs and GPI-anchored proteins are first delivered to the basolateral membranes and then internalized by a clathrin-independent pathway that leads to the apical membrane.145 Thus in some polarised cells the sorting of newly synthesized GSLs and GPI-anchored proteins might not occur at the trans-Golgi, but during internalization through caveolae from the basolateral membrane. Transcytosis is stimulated by cAMP, which requires dihydroCer synthase activation, probably functional to sphinganine removal.146 In agreement, the accumulation of sphinganine results in frustrated cAMP-stimulated transcytosis and impaired apical membrane biogenesis.146
Conclusion
Cells regulate the localization of sphingolipids through a complex compartmentation of sphingolipid metabolism as well as a spectrum of intramembrane and intermembrane transport processes. These are sophisticated processes, controlled by local or extracellular interactions and stimuli and strictly interconnected, endowing the cells to fully integrate different sphingolipid molecules as key components and modulators of cell structure and functional properties. This complexity appears fundamental in sphingolipid transport, making it efficient, allowing it to move single or pools of sphingolipid molecules even in opposite directions and to proceed at different rates and adapting sphingolipid localization to specific inputs. In cells, this complexity should allow different sphingolipids to behave differently under different conditions, to respond to different cellular requirements and finally to display multiple functional properties.
In spite of extensive work, in most cases the precise nature of the molecular mechanisms that control the interaction between sphingolipids and their membrane environment and govern all the specific processes of sphingolipid transport in the living cells remains limited. Several important questions about its complexity remain also unanswered, such as the mechanisms underlying the interactions of multiple systems involved in sphingolipid transport, the control of sphingolipid transport by molecular interactions and signaling mechanism, as well as the pathological consequences of its derangement.
Solving these questions will represent an important challenge in the future. This will require the development of improved, new methods for manipulation and detection of specific molecular species or pools of sphingolipids at distinct intracellular sites, as well as an integrated approach that also includes the transport of membrane proteins.