Abstract
Sphingolipids are a family of ubiquitous membrane components that exhibit multiple functional properties fundamental to cell properties. Sphingolipid transport represents a crucial aspect in the metabolism, signaling and biological role of sphingolipids. Different mechanisms of sphingolipid movements contribute to their selective localization in different membranes but also in different portions and sides of the same membrane, thus ensuring and regulating their interaction with different enzymes and target molecules.
In this topic we will describe the knowledge of the different mechanisms of sphingolipid movements within and between membranes, focusing on the recent advances in this field and considering the role played by selective sphingolipid molecules in the regulation of these mechanisms.
Introduction
Sphingolipids are a family of amphipathic lipids that exhibit extraordinary structural and multiple functional properties fundamental to cell characteristics. They are ubiquitous membrane components, being present in different organelle membranes and particularly abundant in the plasma membrane (PM). In biological membranes, sphingomyelin (SM) and glycosphingolipids (GSLs) represent the major sphingolipids, these display an asymmetric or polarized distribution and play important roles in the regulation of membrane fluidity and sub-domain structure. Their physical-chemical properties enable them to fulfil and regulate a large spectrum ofrelevant biological functions such as molecular sorting, cell-cell interaction and intracellular transport. Moreover, different intermediates of sphingolipid metabolism, as sphingosine (Sph), sphingosine-1-phosphate (S1P), ceramide (Cer) and ceramide-1-phosphate (Cer1P), can act as bioactive molecules involved, as intra- or extracellular messengers, in the regulation of crucial processes as cell growth, death, adhesion, migration and senescence. A crucial and fundamental aspect in the spectrum of the biological functions of sphingolipids is represented by their intra- and extracellular transport. Indeed, the complex compartmentation of sphingolipid metabolism, the specific sphingolipid composition of different cells and organelles and the proper localization of selected sphingoid molecules, functional to the interaction with multiple targets in different locations, are strictly dependent from and regulated by different modalities of sphingolipid transport.
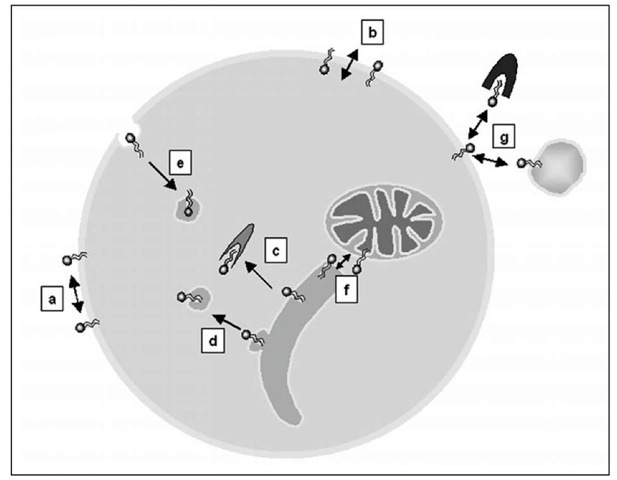
Sphingolipid transport has emerged as a sophisticated and complex tool crucial in moving, transferring and finally locating the different sphingolipids in a specific subcellular site or in the extracellular milieu. This transport involves short-distance movements within a membrane (in-tramembrane transport), including lateral and transmembrane motions and trafficking between different membranes (intermembrane transport), including that mediated by proteins and vesicle flow (Fig. 1).
Figure 1. Different types of sphingolipid transport. Sphingolipids can display movements within a membrane, including lateral diffusion (a) and transbilayer motion (b). Sphingolipid transport between different cellular membranes can be protein-mediated (c) and vesicle-mediated, including vesicles of the biosynthetic route (d) and endocytotic vesicles (e). Sphingolipids could be exchanged as monomers between the cytosolic layer of organelle membranes (f). Plasma membrane sphingolipid may be exported through the interaction with specific extracellular protein/lipoproteins (g). For simplicity, the possible involvement of protein transporters in (b) and (g) is omitted. Most of these movements may occur at or between different cellular membranes.
Intramembrane Sphingolipid Movements
The behaviour of sphingolipids in a membrane represents a crucial aspect of their transport as well as biological properties. In this section we will first analyze the dynamics and organization of sphingolipids within a membrane layer and then the mechanisms underlying the translocation of sphingolipid molecules between the two leaflets of a biological membrane.
Lateral Diffusion and Lateral Phase Separation of Sphingolipids
Sphingolipids, as the other lipid components of cell membranes, are characterized by a high diffusion rate in a leaflet of the bilayer. The lateral diffusion of different sphingolipids in model and biological membranes has been evaluated by different techniques, which include spectro-scopic methods such as fluorescence energy transfer, fluorescence quenching, including the monomer to excimer transition, diffusion measurements based on fluorescence recovery after photobleaching (FRAP), fluorescence correlation spectroscopy, single particle tracking (SPT) and NMR spectroscopy. The reported lateral diffusion coefficient of different sphingolipids ranges from 10~7 to 10~10 cm2/sec, this variability is most likely due to the different methods employed. Studies performed with pyrene-labeled sphingolipids report that, in a fluid bilayer (i.e., in a liquid disordered phase), the diffusion coefficient of SM, glucosylceramide (GlcCer) and different gangliosides is similar (about 1.6 x 10~7cm2/sec)1,2 and comparable to that of phosphatidylcholine (PC) obtained with the same spectroscopic approach.3 Using gangliosides differently labelled at the polar head, the diffusion values determined with FRAP is in the range of 10~9 cm2/sec.4,5 From these data, it emerges that the lateral diffusion of a lipid in the bilayer is primarily determined by the hydrophobic membrane-bound portion of the molecule and does not significantly differ between glycero- and sphingolipids. Due to their high diffusion rate, sphingolipids should explore an area of 0.1-1 ^m2/sec in a cell membrane and should display a homogeneous distribution in the membrane plane. However, a great amount ofevidence has been presented indicating that lateral sphingolipid segregation in a membrane plane may occur. The existence in living cells of sphingolipid-enriched domains, functional to lipid sorting to the apical membrane was initially postulated by Simons and Van Meer.6 Subsequently it was found that the GPI-anchored protein can be sorted through the same pathway.7 The isolation from biological membranes at low temperature of detergent-insoluble sphingolipid-cholesterol (Chol) enriched domains containing GPI anchored proteins8 was the basis for a large body of work addressing the composition, organization and function of these structures in living cells. Lateral phase separation of sphingolipids and the tendency of Chol to associate to sphingolipid-enriched domains have been demonstrated in model membranes by different studies (reviewed in refs. 9-11). Studies performed with ternary mixtures of PC, SM/GSL and Chol indicate that two distinct physical states can coexist in the fluid state in a wide range of temperatures and lipid composition. The physical state of sphingolipid-Chol enriched domains has been defined as a liquid ordered state (Lo) and differs from that of the bulk lipid bilayer described by the more fluid liquid disordered state (La).12-14 In the Lo state the acyl chains exist in a highly ordered conformation which results in a reduction of the area per lipid, a tight lipid packing and a greater thickness of the bilayer. The intermolecular forces involved in the phase segregation of sphingolipids and Chol include lipid-lipid hydrogen bonds, in which sphingolipids can act both as acceptors and donors, weak dipolar interactions between sphingolipid polar heads and van der Waals interactions. The preferential partitioning of Chol in these domains can also involve the model of hydrophobic shielding or the ‘umbrella effect’ in which the steric hindrance of strongly hydrated sphingolipid polar heads may favour the Chol insertion in the bilayer. All these considerations attain to a structural and static description of sphingolipid-Chol enriched lipid domains, but how is the lateral diffusion of sphingolipids in such structures ? In phospholipid/Chol systems, pulsed field gradient NMR technique15 allowed to estimate the diffusion rate of a lipid in the Lo phase is about 3-5 fold slower than in a liquid disordered one, but not different enough to impede lipid exchange between the two phases.1,15 When both SM and Chol were present in the membrane model, the diffusion coefficients were compatible with that determined in more simple systems, but in contrast to the latter, the lipid exchange between the Lo and the La phases was slower.17 These findings are in agreement with the results obtained in single particle tracking, which demonstrated a transient confinement of the 4-fold slower GM1 in a limited membrane portion.18 Thus the physical state (i.e., Lo vs disordered state) is not the only determinant for the lipid dynamic in the domain. The hydrophobic mismatch between the thicker sphingolipid-enriched domain and the thinner surrounding bilayer, which contributes to the energy required to lipid domain formation and size,19 can partially contribute to explain the confined diffusion of lipids in coexisting Lo and disordered phases.
Sphingolipid Raft Dynamics
The raft concept, originally hypothesized for the specific sorting of sphingolipids and proteins in the PM,6,7 has been proposed as a key element in almost all the forms of vesicular traffic.20,21 The majority of information about the composition and function oflipid rafts have been obtained on the basis of the operational definition of rafts as detergent-resistant membrane fraction at low temperature, which implies that stable lipid rafts do exist in biological membranes. Nevertheless, the existence, dimension, lifetime and ultimately the physiological function of sphingolipid-Chol enriched domains in living cells are a matter of great debate among the researchers in the field.
This controversy mainly comes from the difficulty to visualize rafts in living cells. Apart from caveolae, a subset of lipid rafts which can be easily detected by electron microscopy as about 100 nm-sized structures, the direct observation of rafts by optical microscopy has turned out to be impossible, thus suggesting that native rafts are smaller than the optical diffraction resolution limit of 300 nm. On the other hand, the sizing of these structure by indirect methods postulates raft dimensions ranging from 10 to 700 nm,9,22 thus suggesting the possibility that the observed larger structures represent macrodomains derived from the aggregation/coalescence ofnanometer scaled rafts. This implies that native membrane rafts are highly dynamic, heterogeneous and short lived structures. In the 2006 Keystone symposium on lipid rafts and cell functions, the biophysicists, biochemists and cell biologists converged on the following definition "Membrane rafts are small (10-200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes. Small rafts can sometimes be stabilized to form larger platforms through protein-protein and protein-lipid interactions".23 Different aspects can contribute to the metastability of small nanoscaled rafts in the membranes, as (i) the compartmentalization into small domains through actin-based membrane skeleton fence, with integral proteins as barrier palisade; and (ii) the lipid and protein heterogeneity of biological membranes, which limits the hydrophobic mismatch between thicker sphingolipid-enriched domains and the more thinner surrounding bilayer (reviewed in ref. 24).
The dynamic evolution of small membrane rafts into larger structures can be induced by ligand binding as, for example, the cholera toxin binding to GM125 and the EGF binding to its receptor, which induces the merging oftwo types ofGM1-containing small domains.26 In addition, Cer strongly associates with lipid rafts27,28 and can induce the coalescence oflarge domains in PM, thus facilitating the raft-residing CD95/Fas death receptor clustering and the subsequent induction of apoptosis.29 The Cer role in membrane lateral organization and dynamics has been extensively reviewed.30-32 The formation of membrane domains highly enriched in Cer can be relevant to different physiological processes. First, it can contribute to the definition ofsignaling platforms generated by the activity of a signaling-related sphingomyelinase (SMase). Second, Cer can displace Chol from membrane rafts, as both compete in their association with lipid rafts due to the limited capacity ofsphingolipids to shield the small polar groups of Cer and Chol from contact with water.30,32 This can be at the basis of the Chol displacement from PMs upon Cer generation, which may affect membrane Chol homeostasis and may have implications for the association and activity of Chol-bound proteins. Finally, the formation of Cer-enriched microdomains can promote membrane vesiculation and vesicle budding. This has been observed in PC/SM giant unilamellar vesicles, in which Cer-enriched microdomains were rapidly formed after treatment of vesicles with SMase and can also occur in erythrocytes after loading with high concentrations of C18-Cer.33 The Cer-mediated vesicle budding has been implicated in the sorting of subcellular membranes into different populations of intracellular vesicles34 and in the ER-Golgi vesicular traffic of both sphingolipids and proteins.35
Transbilayer Transport
The transfer of sphingolipid molecules from one to the other leaflet of a biological membrane represents a further, intriguing aspect of sphingolipid movement. This movement may be of relevance to different processes, such as the formation and maintenance of a proper sphingolipid asymmetry, thus being important in the PM and organelle structure/functional properties and in the mechanisms of intracellular sphingolipid trafficking. It may also enable the sphingolipid molecule to interact with a protein/enzyme that resides on the opposite side of the membrane that must be crossed, thus being relevant in sphingolipid metabolism and signaling. In addition, it may be crucial to the interaction of a sphingolipid with an extracellular protein/lipoprotein and thus to its export into the extracellular milieu.
As for other amphipatic lipids, the transbilayer movement of sphingolipids can be from the outer to the inner monolayer of a membrane ("flip"), in the opposite direction ("flop"), or bi-directional (scrambling or "flip-flop"). All these motions can occur spontaneously or facilitated by the transport/interaction with a protein.
The spontaneous behaviour of a (sphingo)lipid molecule within a membrane is influenced by different factors, including the balance between its hydrophobic tail and polar headgroup, the composition of both the sphingolipid alkyl chain and the membrane. It is generally accepted that sphingolipids with a reduced or abundant polar headgroup will display fast or slow spontaneous transbilayer movements, respectively. Since most sphingolipids possess a bulky headgroup, they exhibit the tendency to reside in a membrane monolayer for long periods of time. In agreement, the studies on the spontaneous transbilayer diffusion of spin-labelled SMs and gangliosides in model membranes have shown that this exchange does not spontaneously occur or, if it does it, is very slow.36-38 On the opposite, simple sphingolipids such as GlcCer and Cer can move between leaflets.33,39,40
Cellular membranes, particularly eukaryotic PMs, are equipped with specialised proteins that can either actively translocate lipids from one leaflet to the other or facilitate a passive equilibration of lipids between the two membrane halves. Protein transporters are referred as flippases (mediating transport from outer, noncytosolic, to inner, cytosolic, layer), floppases (from inner to outer layer) and scramblases (facilitating flip-flop, i.e., nonspecific redistribution across bilayers). Increasing evidence supports that different subfamilies ofATP binding cassette (ABC) transporter family contribute to, or are involved in, sphingolipid passage across a membrane (Table 1).41 ABC proteins have been generally recognized as drug efflux pumps that protect the body from various toxic substances. However, they exhibit scarce selectivity and are able to move various molecules against their concentration gradients.42,43 After its binding to the Nucleotide Binding Domain (NBD), ATP provides the energy required to actively transport substrates across the membrane. In eukaryotes, most ABC transporters contain two hydrophobic domains, each with 6-11 membrane-spanning a-helices and two NBDs exposed to the cytoplasm. Some ABCs contain only one transmembrane domain and one NBD, but can combine into homo- or hetero-dimers to form functional transporters. In mammals, ABC transporters are expressed in PM and different intracellular membranes, including those of endoplasmic reticulum (ER), Golgi, mitochondria, endosomes, lysosomes and exocytotic vesicles and almost all the eukaryotic ABC proteins studied to date are floppases, transporting substrates outwardly from cytosol into organelles or out of cells. On the bases of their sequence homology and NBD organization, human ABC transporter have been classified into seven subfamilies, designated A-G.
Notably, since the expression of ABC transporters may be restricted in a cell/tissue-specific fashion, different ABC expression may contribute to differences in sphingolipid transport and thus to cell/tissue sphingolipid pattern and functional specialization.
Transbilayer Transfer of Sphingomyelin and Complex Glycosphingolipids
The distribution of complex sphingolipids (SM and GSLs) across PMs is highly asymmetric, being present essentially in the outer monolayer. This asymmetry appears to be dictated by the exocytotic traffic of newly synthesized sphingolipids and maintained with low levels of flip-flop across the bilayer. In fact, SM and GSLs are synthesized from Cer and GlcCer on the luminal side of the Golgi, where they cannot translocate to the cytosolic face. Upon exocytosis, they reach the external leaflet of the PM, where little or no transport to the cytosolic face occurs under basal conditions. However, although in most cells the great majority (>80%) of SM is normally confined to the outer leaflet of the PM and to the luminal face (corresponding to the outer one) of organelle membranes, in some membranes the percentage of SM present in the outer leaflet is markedly reduced. For example, SM in the outer leaflet accounts for only 65% of intestinal brush border membranes and hepatocytes, possibly reflecting specific functional properties of these membranes.44 In liver ER and brain microsomes, SM facing the luminal face accounts for 60%, being thus markedly lower than in other organelles.44
As biogenic (self-synthesizing) membrane, the ER membrane requires phospholipid scrambling for bilayer assembly and maintenance. Different studies reported that phospholipid scrambling in ER occurs very rapidly, bi-directionally, independently ofthe phospholipid head group and requires specific membrane proteins. Indeed, the transmembrane movement of SM in ER is significantly faster than in other membranes, although much slower than glycerophospholipids.
Table 1. ABC transporters involved in sphingolipid transport
|
Transporter |
Proposed Role |
Membranes/Cells |
ABC Localization |
Notes |
Deficiency-Associated Disease |
|
ABCA1 |
SM floppase |
Model membranes |
PM |
Transports mainly cholesterol and PC |
HDL deficiency |
|
ABCA1 |
S1 P floppase |
Astrocytes Blood brain barrier |
PM |
||
|
ABC-like |
S1 P floppase |
Platelets |
PM |
Inhibited by glyburide |
|
|
ABCA2 |
Transport of SM and gangliosides ?? |
Oligodendrocytes, some neurons |
Lvsosome, endosome, Colgi, PM |
SL traffic during SL biosynthesis or degradation |
Expression correlated with Alzheimer’s disease |
|
ABCA7 |
Cer transport ?? |
Keratinocytes |
Lamellar granules |
Facilitates Cer production at the ER |
|
|
and traffic from/to lamellar granules |
|||||
|
ABCA12 |
ClcCer floppase |
Keratinocyte Lung |
Colgi, lamellar granules |
Mediates transport into lamellar granules |
Harlequin ichthyosis |
|
ABCB1 |
SM, CSL floppase |
Blood brain barrier |
PM |
Defective multidrug resistance |
|
|
(P-gp, MDR1) |
in cancer |
||||
|
ABCC1 (MRP1) |
SM, ClcCer floppase |
Pig kidney-derived polarized cells |
PM |
Defective multidrug resistance in cancer |
|
|
ABCC1 (MRP1) |
S1 P floppase |
Mast cells, HUVEC |
PM |
Defective multidrug resistance |
|
|
in cancer |
|||||
|
ABCC7 (CFTR) |
S1 P flippase |
Resistance arteries; epithelial cells |
PM |
CF channel |
Cystic fibrosis |
|
ABCC1 |
SM floppase |
Different cells |
PM |
Cholesterol and SM floppase |
Pulmunary lipidosis |
|
ABCC2 (BCRP) |
Cer floppase |
Placental trophoblasts |
PM |
Fetal growth restriction |
Ofrelevance, the transbilayer movement of complex sphingolipids (mainly shown for SM) can occur in and appears functional to, different processes. At the PM, the transbilayer asymmetry of SM can be compromised, along with that of glycerophospholipids, in several physiological and pathological conditions such as neutrophil activation, blood coagulation, apoptosis and suicidal death of erythrocytes (eryptosis).45,46 Indeed, the asymmetric distribution of all the major phospholipid classes, including SM, is rapidly lost upon a persistent increase of cytoplasmic Ca2+, caspase activation, or oxidative stress.47,48 Increased cytosolic Ca2+ and enhanced Cer levels lead to membrane scrambling too and subsequent phosphatidylserine exposure.
A phospholipid-scramblase, isolated from erythrocytes and cloned, has been implicated in the fast bi-directional phospholipids transport between the two monolayers.50 This transporter, named phospholipid scramblase 1 (PLSCR1), is a 37-kDa membrane protein with a short C-terminal external sequence, a single transmembrane segment and a long cytoplasmic extension containing a calcium-binding segment and is expressed in multiple human tissues and cancer cell lines. Ca2+-induced lipid scrambling is defective in cells from patients with Scott syndrome, a bleeding disorder originating from a reduced ability of activated platelets to expose procoagulant phos-pholipids on their surface.51 Intriguingly, in lymphoid cells from a patient with Scott syndrome, scramblase is induced normally during apoptosis, but cannot be activated by Ca2+, suggesting that apoptosis and Ca2+ operate through different pathways to activate the same scramblase.52 More recent reports suggest that different proteins are responsible for scramblase activity and phospholipid scramblases emerged as a group ofhomologous proteins conserved in all eukaryotic organisms and exhibiting different cell expression and subcellular localization.53 Although a certain amount of evidence supports the role of scramblase(s) in destroying PM phospholipid asymmetry at critical cellular events like activation, injury and apoptosis, the function of PLSCR1 as a phospholipids translocator has been recently challenged.53 At present, the role of different scramblases in sphin-golipid transport remains to be clarified.
A further mechanism, consisting in the SMase-induced asymmetric increase in Cer concentration has been proposed as a crucial one in to facilitate the scrambling of other lipids. The observation that a Cer asymmetric generation can promote lipid scrambling (see above) has led to postulate that endogenous SMase could be a lipid scramblase.54
In lipid vesicles, ganglioside GM3 can undergo spontaneous flip-flop as a consequence of SMase-induced Cer on the outer leaflet.55 So far, no evidence has been provided on ganglio-sides scrambling during cell activation and apoptosis. Interestingly, PS exposure on the outer membrane leaflet during cell activation and apoptosis colocalizes with GM1, possibly at the level of lipid rafts.49,56
A further mechanism of complex sphingolipid transfer across the membrane bilayers involves ABC family proteins. Different members of the ABC family have been implicated in facilitating the transport of SM and gangliosides at the PM (Table 1). This transport might be functional not only in maintaining and restoring sphingolipid asymmetry, as example after cell activation, but also in the export of complex sphingolipids to acceptor proteins/lipoproteins.
ABCA1 and ABCG1, two ABC proteins required for lipoprotein generation, appear to be involved in SM outward transport. ABCA1 is able to transfer phospholipids and Chol to lipid-free apoA-I and lipidated apoE. In liposomes made of SM or PC, purified ABCA1 shows high ATPase activity, suggesting that it can recognize phospholipids with choline head groups.57 However, when cellular SM content is reduced, apoA-I-dependent Chol efflux by ABCA1 increases, suggesting that ABCA1 preferentially transports PC and Chol.57 In contrast, ABCG1, a floppase important in the efflux of excess Chol from peripheral tissues to pre|3-HDL and HDL, appears to preferentially recognize Chol and SM as substrates to transport across the PM and secrete.58 ABCG1 stimulates Chol and SM efflux to HDL2 or HDL-3.58 Interestingly, Chol efflux by ABCG1 is strictly dependent on the cellular SM level and this level correlates with SM efflux.59
Recent data also implicate a role of ABCA2, a transporter involved in lipid movement to generate the myelin sheath, as a possible transporter of complex sphingolipids. In the ABCA2 null mice, Sakai et al60 reported several major alterations in the lipid composition of both whole brain and purified myelin fractions. Analysis of brain and myelin lipids revealed selective deficiencies of SM, whereas brain gangliosides and myelin GM1 were significantly increased.60 Since ABCA2 is localized to late endosome/lysosomes and Golgi, it might be involved in the metabolism of neural SM and/or gangliosides.
Some studies provided evidence that ABCB1 (P-gp, MDR1) and ABCC1 (MRP1) can transport short-chain fluorescent SM (and GlcCer, PC) analogs,61,62 implicating these proteins in sphingolipid distribution between the leaflets of the PM. However, the role of these ABC transporters in the movement of natural sphingolipid molecules among biomembranes remains to be elucidated.
Transbilayer Transfer of Monohexosylsphingolipids
In contrast to more complex sphingolipids, the monohexosylsphingolipids GlcCer and ga-lactosylceramide exhibit a spontaneous tendency to move between membrane bilayers. In model membranes, spin-labeled GlcCer and galactosylceramide analogues can spontaneously cross the bilayer by passive diffusion.38 Of relevance, this movement was observed also in the PM of eryth-rocytes and hepatocyte-like cells, as well as in the ER and Golgi membranes.38,63 In both model and plasma membranes, the spontaneous transbilayer movement exhibited a slow rate (half-time between 2 and 5 h at 20′C). However, in rat liver ER and Golgi membranes, the transverse diffusion of spin-labeled monohexosylsphingolipids was much faster (half-time of about 3 min at 20′C), displayed a saturable behaviour, was sensitive to proteases and occurred in both directions,38 indicating that one or more specific scramblase(s) facilitates this rapid movement. This should promote a symmetric distribution of monohexosylsphingolipids across Golgi membranes, allowing their rapid access to the outer (noncytosolic) leaflet, where further glycosilations occurs. Using fluorescent short chain analogues, it has been reported that ABCB1 is involved in GlcCer translocation in the Golgi.63 However, a recent study suggests that P-glycoprotein selectively translocates such analogues but not natural long chain lipids.64
Notably, the transbilayer movement of lactosylceramide has not been detectable in both artificial and natural membranes,38 implying that once synthesised in the Golgi (or produced in the lyso-some), it remains at the lumenal leaflet where it undergoes subsequent metabolic processing.
Recent studies have revealed a mechanism of GlcCer transport specific for epidermal kerati-nocytes and functional to the extracellular formation of Cer. This transport involves ABCA12 transporter in the delivery of GlcCer to secretory granules (termed lamellar granules) in the epidermal keratinocytes.65 Notably, ABCA12 selectively transports long chain GlcCer, essential to the generation of a specific class of ceramides crucial to the correct formation of the epidermal permeability barrier.66 Mutations in ABCA12 result in the failure to deliver GlcCer to lamellar granules and consequent failure to form lamellar bodies and extracellular lamellar membranes67 and underlie harlequin ichthyosis (HI) and lamellar ichthyosis, two devastating skin disorders.65,67 Of relevance, corrective transfer of the ABCA12 into HI keratinocytes restores normal GlcCer loading into lamellar granules.65
Transbilayer Transfer of Ceramide and Sphingoid Bases
Unlike other sphingolipids, Cer and the sphingoid bases Sph and sphinganine are characterised by the presence of a very small polar headgroup, suggesting they can easily undergo transbilayer diffusion. Indeed, different studies reported their rapid scrambling.33,40,68 The reported half-times of Cer flip-flop are less than 1 min at 37′C for the natural C16-Cer in giant vesicles and in the erythrocyte PM.33 This rapid scrambling should give Cer the ability to reside in both sides of the membrane, thus forming membrane spanning domains. Up to now, it is not known if the lipid/ protein composition of different biological membranes might influence the Cer flip-flop. This could be of relevance in the metabolism and functional properties of Cer.
Cer acts as intracellular messenger of different stimuli in multiple cell types and Cer generation appears closely linked to scrambling in both apoptosis and eryptosis.46,69 Intriguingly, recent studies reported that Cer itself can be responsible for the transbilayer motion of phospholipids.33,54,70 The conversion of SM into Cer on only one side of a lipid vesicle appears to generate a surface tension that in turn can generate a surface asymmetry in the bilayer.54 This asymmetry in turn promotes phospholipid scrambling across the PM in a nonspecific fashion. Notably, dihydroceramide, the Cer precursor unable to exert many of the physiological effects of Cer, does not induce phospho-lipid scrambling.
Unexpectedly, some ABC proteins appear to be required also for the efficient translocation of Cer and long chain sphingoid bases (LCBs). It was found that during terminal keratinocyte differentiation, the upregulation of ABCA7 is paralleled by intracellular and surface Cer levels and overexpression of ABCA7 in HeLa cells results in increased levels of intracellular and surface Cer.71 In addition, in placenta trophoblasts, ABCG2 has been proposed as transporter of Cer to the outer leaflet of the PM, protecting placenta from Cer-induced apoptosis.
A novel transporter, referred as Rsb1p (resistant to sphingoid bases) and localized to the PM, was shown to be involved in the export of dihydrosphingosine and phytosphingosine in Saccharomyces cerevisiaeP,74 Intriguingly, Rsbl expression is enhanced in cells with altered glyc-erophospholipid asymmetry due to the disruption of either the inward or outward movement of glycerophospholipids,74 suggesting that altered membrane asymmetry can trigger Rsbl expression. Interestingly, Pdr3p, a transcription factor crucial to multidrug resistance, was shown to activate Rsbl transcription, suggesting that this is functional to LCBs detoxification in the retrograde response of Saccharomyces cerevisiaeP It will be interesting to evaluate whether a floppase exporting LCBs may exist in mammals as well.
Transbilayer Transfer of Sphingosine-1-Phosphate
Unique among sphingoid molecules, S1P exhibits the peculiar property of binding to specific cell surface receptors. Since most S1P is generated intracellularly, S1P transport across the PM is crucial for its action as first messenger. Different cells have been shown to release newly synthesized S1P into the extracellular milieu and such release could be important for the local action of S1P in the extracellular space.76-78 Of note, the extracellular release of S1P appears cell specific, as not all cells exhibit the capacity to excrete S1P.
S1P release can occur constitutively, or can be induced by different stimuli, such as PKC activation, Ca2+ and growth factors.76-78 However, the mechanism of S1P transmembrane traffic is still unclear. With its polar head group, S1P presumably cannot readily permeate the lipid bilayer, its export/secretion into the extracellular space most probably requiring specific transporters. Increasing evidence suggests that members of the ABC transporter family are of relevance for the S1P export out of cells (Table 1). It was shown that export of S1P from activated mast cells is mediated by ABCC179 and the member of the A subfamily ABCA1 is critical for S1P release from astrocytes and at the level of the blood brain barrier.80 Similarly, it was suggested that S1P secretion from HUVEC is also mediated by ABCA1 and ABCC1.81
Some pieces of evidence indicate the involvement of ABC transporters also in the active S1P uptake from the extracellular milieu. In particular, the cystic fibrosis transmembrane regulator ABCC7 has been implicated in the uptake of extracellular S1P by smooth muscle cells,82,83 suggesting that shuttling from the outside to the inside by ABCC7 participates to the regulation of S1P availability for its receptors.
Although all these studies suggest the involvement of different mechanisms in the transbilayer transport of sphingolipid and support the relevance of this movement, the mechanisms underlying it remain largely unknown and further intensive studies are necessary to elucidate the players, localisation and regulation of these mechanisms.