1. Introduction
Two-dimensional gel electrophoresis (2DE) with immobilized pH gradients (IPGs) combined with protein identification by mass spectrometry (MS) is currently the workhorse for proteomics. In spite of new promising technologies that have emerged (MudPIT, stable isotope labeling, arrays) (see Article 13, Multidimensional liquid chromatography tandem mass spectrometry for biological discovery, Volume 5, Article 23, ICAT and other labeling strategies for semiquantitative LC-based expression profiling, Volume 5, and Article 24, Protein arrays, Volume 5), 2DE is currently the only technique that can be routinely applied for parallel quantitative expression profiling of large sets of complex protein mixtures such as whole-cell lysates.
Whatever technology is used, proteome analysis is technically challenging, because the number of different proteins expressed at a given time under defined biological conditions is likely to be in the range of several thousand for simple prokaryotic organisms up to at least 10 000 in eukaryotic cell extracts. Moreover, current proteomic studies have revealed that the majority of identified proteins are abundant “housekeeping” proteins that are present in numbers of 105 to 106 copies per cell, whereas proteins such as receptor molecules that are present in much lower concentrations (typically <100 molecules per cell) are usually not detected. Consequently, improved methods for enrichment of low-abundance proteins are required, such as prefractionation procedures, as well as more sensitive detection and quantitation methods.
2DE couples isoelectric focusing (IEF) and sodium dodecyl sulfate polyacry-lamide gel electrophoresis (SDS-PAGE) to resolve proteins according to two independent parameters, that is, isoelectric point (pI) in the first dimension and molecular mass (Mr) in the second. Depending on the gel size and pH gradient used, 2DE can resolve more than 5000 proteins simultaneously (~2000 proteins routinely), and can detect < 1 ng of protein per spot. Furthermore, it delivers a map of intact proteins, which reflects changes in protein expression level, isoforms or posttranslational modifications. This is in contrast to LC-MS/MS-based methods, which perform analysis on peptides, where Mr and pI information is lost, and where stable isotope labeling is required for quantitative analysis.
The former limitations of carrier ampholyte-based 2DE (O’Farrell, 1975) with respect to reproducibility, resolution, separation of very acidic and/or very basic proteins, and sample loading capacity have been solved by the introduction of IPGs for the first dimension of 2DE (Gorg et al., 1988). Narrow-overlapping pH gradients provide increased resolution (ApI = 0.001) and detection of low-abundance proteins, whereas alkaline proteins up to pH 12 have been separated under steady state conditions (Gorg et al., 2000; Wildgruber et al., 2002; Drews et al., 2004).
The analysis of very hydrophobic proteins such as integral membrane proteins remains a challenge for both 2DE and LC-based proteomic approaches. Currently, the best strategy is the combination of SDS-PAGE analysis of membrane fractions in combination with LC-MS/MS. This method has been termed geLC-MS/MS (Li et al., 2003).
2. 2DE-MS proteomic workflow
The major steps of the 2DE-MS workflow are
• sample preparation/solubilization
• protein separation by 2DE
• protein detection and quantitation
• computer-assisted analysis of 2DE patterns
• protein identification and characterization
• 2D protein database construction.
and will be discussed in the following sections.
Sample preparation should be as simple as possible to increase reproducibility. Although many “standard” protocols for sample preparation have been published, these protocols must be optimized for the type of sample to be analyzed. Some general recommendations, however, can be made: protein modifications during sample preparation must be minimized, because they might result in artifactual spots on 2D gels. In particular, proteolytic enzymes in the sample must be inactivated. Samples containing urea must not be heated to avoid charge heterogeneities caused by carbamylation of the proteins by isocyanate formed in the decomposition of urea (Dunn, 1993). The three fundamental steps in sample preparation are cell disruption, inactivation and or removal of interfering substances, and solubilization of the proteins (see Article 2, Sample preparation for proteomics, Volume 5).
Cell disruption can be achieved by osmotic lysis, freeze-thaw cycling, detergent lysis, enzymatic lysis of the cell wall, sonication, grinding with (or without) liquid nitrogen, high pressure (e.g., French press), homogenization with glass beads and a bead beater, nitrogen cavitation, or a rotating blade homogenizer. These methods can be used individually or in combination.
During or after cell lysis, interfering compounds such as proteolytic enzymes, salts, lipids, nucleic acids, polysaccharides, and plant phenols have to be inactivated or removed. The two most important parameters are salt and proteolysis. Proteases must be inactivated to prevent protein degradation that otherwise may result in artifactual spots and loss of high Mr proteins. Protease inhibitors are usually added, but they may modify proteins and cause charge artifacts. Other remedies are boiling the sample in SDS-buffer (without urea!), or inactivating proteases by low pH (e.g., precipitating with ice-cold trichloroacetic acid (TCA)). It should be kept in mind that it may be rather difficult to completely inactivate all proteases. TCA/acetone precipitation is very useful for minimizing protein degradation and removing interfering compounds, such as salt. Attention has to be paid, however, to protein losses due to incomplete precipitation and/or resolubilization of proteins. Moreover, a completely different set of proteins may be obtained by extraction with lysis buffer, depending on whether there was a preceding TCA precipitation step. On the other hand, this effect can be used for the enrichment of very alkaline proteins (such as ribosomal or nuclear proteins) from total cell lysates (Gorg et al., 2000).
Salt ions may interfere with electrophoretic separation and should be removed if their concentration is too high (>100mM). Removal can be achieved by precipitation of proteins with TCA or organic solvents (e.g., cold acetone). One alternative is the use of 2D cleanup kits (e.g., Amersham Biosciences). Another is dilution of the sample below a critical salt concentration followed by application of a larger sample volume onto the IPG gel. The sample is “desalted” in the gel by using low voltage at the beginning of the run.
After cell disruption and/or removal of interfering compounds, the individual polypeptides must be denatured, solublized, and reduced to disrupt intra- and intermolecular interactions while maintaining the inherent charge properties. The most popular sample solubilization buffer is based on O’Farrell’s lysis buffer and modifications thereof (O’Farrell, 1975) (9 M urea, 2-4% CHAPS, 1% dithiothreitol (DTT), 2% (v/v) carrier ampholytes). Unfortunately, urea lysis buffer is not ideal for the solubilization of all protein classes, particularly for membrane or other highly hydrophobic proteins. Improvement in the solubilization of hydrophobic proteins has come with the use of thiourea and new zwitterionic detergents such as sulfobetaines (Luche et al., 2003).
3. Prefractionation procedures
Prefractionation can be used to reduce the complexity of the sample, enrich for certain proteins such as low-copy number proteins or alkaline proteins, and to get some information on the topology of the proteins. This can be accomplished by:
• isolation of specific cell types from a tissue, for example, fluorescence activated cell sorting (FACS), or laser capture micro dissection (see Article 6, Laser-based microdissection approaches and applications, Volume 5);
• isolation of cell compartments and/or organelles, for example, by sucrose gradient centrifugation, or free flow electrophoresis;
• selective precipitation of certain protein classes (e.g., TCA/acetone precipitation for ribosomal proteins);
• sequential extraction procedures with increasingly powerful solubilizing buffers, for example, aqueous buffers, organic solvents (e.g., ethanol or chloroform/ methanol), and detergent-based extraction solutions;
• chromatographic or electrokinetic separation methods, such as column chro-matography, affinity purification, electrophoresis in the liquid phase and/or IEF in granulated gels.
4. 2DE with IPGs (IPG-Dalt)
IPGs are based on the use of the bifunctional Immobiline reagents, a series of 10 chemically well-defined acrylamide derivatives with the general structure CH2=CH-CO-NH-R, where R is either a carboxyl or an amino group. These reagents form a series of buffers with different pK values between pK 1 and >12. Because the reactive end of the molecule is copolymerized with the acrylamide matrix, extremely stable pH gradients are generated, allowing true steady state IEF with increased reproducibility, as has been demonstrated in several inter-laboratory comparisons (Corbett etal., 1994; Blomberg etal., 1995).
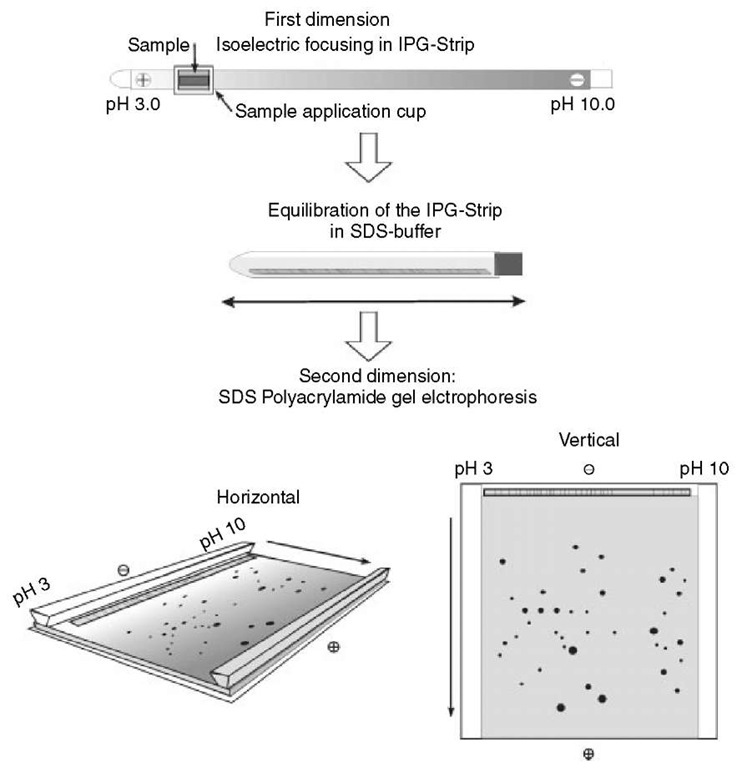
The original protocol of 2DE with immobilized pH gradients (IPG-Dalt) was described by Gorg et al. (1988, updated in 2000 and 2004), summarizing the critical parameters inherent to isoelectric focusing with IPGs and a number of experimental conditions (Figure 1). The first dimension of IPG-Dalt, IEF, is performed in individual, 3-mm wide and up to 24-cm-long IPG gel strips cast on GelBond PAGfilm (laboratory-made or commercial Immobiline Dry-Strips). Samples can be applied either by cup-loading or by in-gel rehydration. For analytical purposes, typically 100 |g of protein can be loaded on an 18-cm-long, wide pH range gradient, and 500 |g on narrow range IPGs. For micropreparative purposes, five to ten times more protein can be applied. After IEF, the IPG strips are equilibrated with SDS-buffer in the presence of urea, glycerol, DTT, and iodoacetamide, and applied onto horizontal or vertical SDS gels in the second dimension (see Article 29, Two-dimensional gel electrophoresis (2-DE), Volume 5).
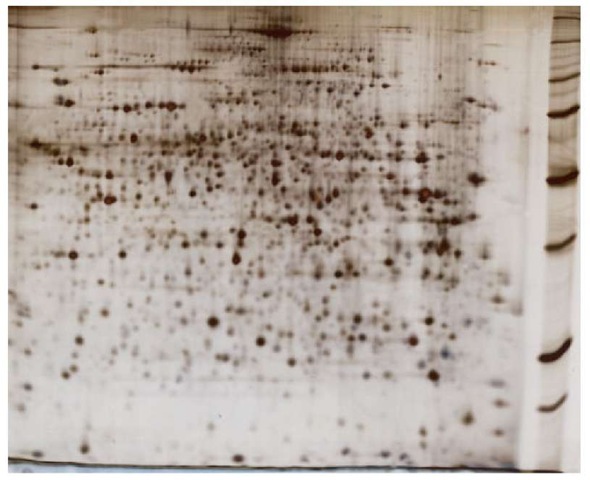
The choice of pH gradient primarily depends on the sample’s protein complexity. Wide IPGs, such as IPG 4-7, 4-9, or 3-11, are used to analyze simple proteomes (small genome, organelle, or other subfraction), or to get an overview of a more complex proteome, respectively (Figure 2).
With samples such as total lysates of eukaryotic cells, 2D electrophoresis on a single wide-range pH gradient reveals only a small percentage of the whole proteome. The best remedy, preferably in combination with prefractionation procedures, is to use multiple narrow overlapping IPGs (“zoom-in” gels, e.g., IPG 4-5, IPG 4.5-5.5, or 5.0-6.0) and/or extended separation distances to achieve an optimal resolution to avoid multiple proteins in a single spot for unambiguous protein identification and to facilitate the application of higher protein amounts for the detection of minor components (Wildgruber et al., 2000; Westbrook et al., 2001).
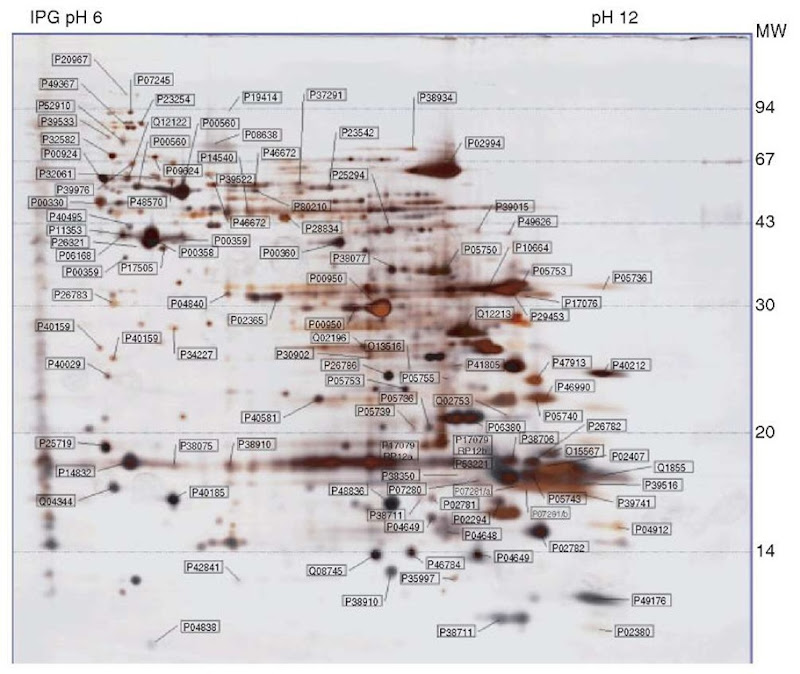
Strongly alkaline proteins such as ribosomal and nuclear proteins with closely related pIs between 10.5 and 11.8 are focused to the steady state by using IPGs 3-12, 6-12, or 9-12 (Wildgruber et al., 2002; Drews et al., 2004) (Figure 3). For optimized separation, cup-loading at the anode is mandatory, and the use of high voltages (final settings up to 8000 V) is recommended. With narrow gradients between pH 7 and 10, horizontal streaking due to DTT depletion can occur at the basic end. To avoid streaking, the cysteines should be stabilized as mixed disulfides by using hydroxyethyldisulfide reagent (DeStreakTM Amersham Biosciences) in the IPG strip rehydration solution instead of a reductant (Olsson et al., 2002).
Figure 1 The principle of 2DE with IPGs according to Gorg et al. 1988, 2000, 2004
4.1. IEF temperature
Spot positions vary along the pH axis with different applied temperatures (Gorg et al., 1991). It is thus very important to run the separations at an actively controlled temperature, where 20°C proved to provide the optimal conditions.
Figure 2 2DE of a TCA-acetone extract of mouse liver proteins, separated by IEF in a 24-cm-long IPG strip containing a nonlinear pH gradient 3-11, followed by SDS-PAGE in a vertical 12.5% gel. Protein detection by silver staining
4.2. Optimization of focusing conditions
Settings are usually limited to 50 |A per strip and 150 V to avoid Joule heating, because the conductivity is initially high due to salts. As the run proceeds, the salt ions migrate to the electrodes, resulting in decreased conductivity and allowing high voltages to be applied. Final settings up to 8000 V are particularly used for zoom-in gels and alkaline pH gradients. The longer the IPG strip and the narrower the pH gradient, the more volt-hours are required to achieve steady state separation for high reproducibility (Gorg et al., 2000; Gorg et al., 2004).
4.3. Storage of IPG strips after IEF
If the second dimension cannot be performed directly after IEF, the IPG strips should be immediately frozen and stored at -70°C between two plastic sheets.
5. Equilibration of IPG gel strips
Before the second-dimension separation, it is essential that the IPG strips are equilibrated to allow the separated proteins to fully interact with SDS. Relatively long equilibration times (10-15 min), as well as urea and glycerol (to reduce electroendosmotic effects) are required to improve protein transfer from the first to the second dimension. The best protocol by far is to incubate the IPG strips for 10-15 min in the Tris-HCl buffer originally described by Gorg et al. (1988) (50 mM Tris-HCl (pH 8.8), containing 2% (w/v) SDS, 1% (w/v) dithiothreitol (DTT), 6M urea and 30% (w/v) glycerol). This is followed by a further 10-15 min equilibration in the same solution containing 4% (w/v) iodoacetamide instead of DTT. The latter step is used to alkylate any free DTT, as otherwise it migrates through the second-dimension SDS-PAGE gel, resulting in an artifact known as point streaking that can be observed after silver staining. More importantly, the iodoacetamide alkylates sulfhydryl groups and prevents their reoxidation; this step is highly recommended for subsequent spot identification by mass spectrometry. After equilibration, the IPG strips are applied onto the surface of the second-dimension horizontal or vertical SDS-PAGE gels.
Figure 3 2DE of a TCA-acetone extract of Saccharomyces cerevisiae proteins, separated by IEF in an 18-cm-long IPG strip containing a linear pH gradient 6-12, followed by SDS-PAGE in a vertical 15% gel. Protein detection by silver staining showing the 106 mapped and identified spots annotated by Swiss-Prot accession numbers
6. Second dimension: SDS-PAGE
SDS-PAGE can be performed on horizontal or vertical systems. Horizontal setups are ideally suited for ready-made gels (e.g., ExcelGel SDS; Amersham Biosciences), whereas vertical systems are preferred for multiple runs in parallel, in particular for large-scale proteome analysis which usually requires simultaneous electrophoresis of batches of second-dimension SDS-PAGE gels for higher throughput and maximal reproducibility. The most commonly used buffers for the second dimension of 2DE are the discontinuous buffer system of Laemmli (1970) and modifications thereof, although for special purposes other buffer systems are employed, such as borate buffers for the separation of highly glycosylated proteins (Patton et al., 1991), or Tris-Tricine buffer systems for the separation of low molecular mass (3-30 kDa) polypeptides (Schagger and von Jagow, 1987). Typically, gel sizes of 20 x 25 cm2 and a gel thickness of 1.0 mm are recommended. In contrast to horizontal SDS-PAGE systems, it is not necessary to use stacking gels with vertical setups, as the protein zones within the IPG strips are already concentrated and the nonrestrictive, low polyacrylamide concentration IEF gel acts as a stacking gel (Dunn and Gorg, 2001).
7. Difference gel electrophoresis (DIGE)
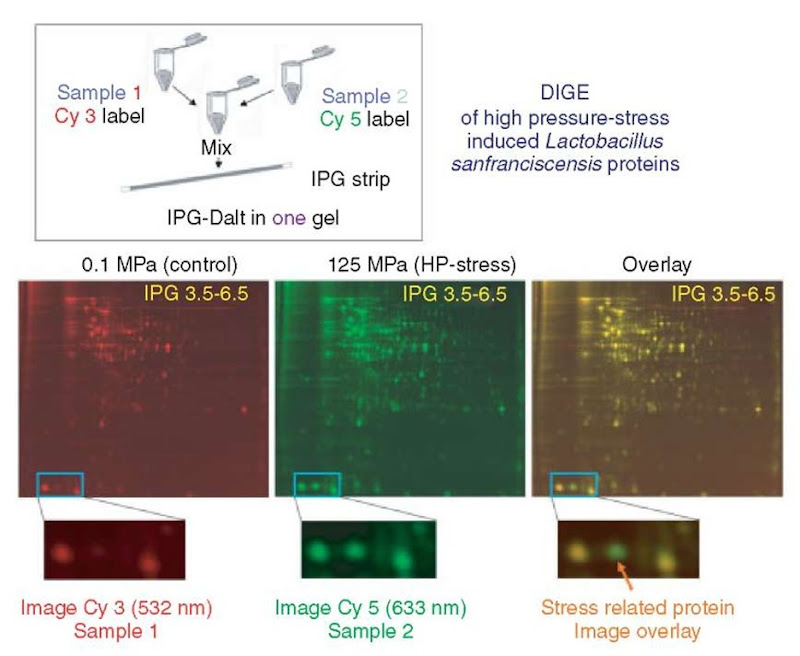
A bottleneck for high-throughput proteomic studies is image analysis. In conventional 2D methodology, protein samples are separated on individual gels, stained, and quantified, followed by image comparison with computer-aided image analysis programs. Because multistep 2DE technology often prohibits different images from being perfectly superimposable, image analysis is frequently very time consuming. To shorten this laborious procedure, Unlu et al. (1997) have developed a method called fluorescent difference gel electrophoresis (DIGE), in which two samples are labeled in vitro using two different fluorescent cyanine dyes (CyDyes, Amersham Biosciences) differing in their excitation and emission wavelengths, then mixed before IEF and separated on a single 2D gel. After consecutive excitation with both wavelengths, the images are overlaid and subtracted, whereby only differences (e.g., up- or downregulated, and/or posttranslationally modified proteins) between the two samples are visualized (Figure 4). Owing to the comigration of both samples, methodological variations in spot positions and protein abundance are excluded, and, consequently, image analysis is facilitated considerably (see Article 25, 2D DIGE, Volume 5 and Article 30, 2-D Difference Gel Electrophoresis – an accurate quantitative method for protein analysis, Volume 5).
A third cyanine dye is now available, which makes it possible to include an internal standard, which is run on all gels within a series of experiments. This internal standard, typically a pooled mixture of all the samples in the experiment labeled with this dye, is used for normalization of data between gels thereby minimizing experimental variation and increasing the confidence in matching and quantitation of different gels in complex experimental designs (Alban et al., 2003). Applications that profit from the DIGE system include the investigation of differential protein expression of samples generated under various prespecified conditions, the comparison of extracts, and the analysis of biological variance. In short, all analyses in which 2D gels need to be compared are simplified and accelerated by this method.
Figure 4 DIGE of high-pressure inducible Lactobacillus sanfranciscensis proteins. Samples (Control (at 0.1 Mpa) and high-pressure stressed (at 125 MPa)) were labeled in vitro with two different fluorescent cyanine dyes (Cy3 and Cy5, respectively) differing in their excitation and emission wavelengths. The samples were mixed, and the mixture was separated on a single 2DE gel. After consecutive excitation with both wavelengths, the resultant gel images were overlayed to visualize differences (e.g., up- or downregulated proteins) between the samples
8. Protein visualization
After 2DE, the separated proteins have to be visualized, either by “universal” or by specific staining methods. The most important properties of stains are high sensitivity (low detection limit), high linear dynamic range (for quantitative accuracy), reproducibility, and compatibility with postelectrophoretic protein identification procedures, such as mass spectrometry (Dunn, 1993; see also Article 27, Detecting protein posttranslational modifications using small molecule probes and mul-tiwavelength imaging devices, Volume 5). Unfortunately, currently no staining method meets all requirements for proteome analysis.
Universal detection methods of proteins on 2D gels include staining with anionic dyes (e.g., Coomassie Blue), negative staining with metal cations (e.g., zinc imi-dazole), silver staining, fluorescence staining or labeling, and radioactive isotopes, using autoradiography, fluorography, or PhosphorImaging (Patton, 2002). For most of these staining procedures, the resolved polypeptides have to be fixed in solutions such as in ethanol/acetic acid/ H2O for at least several hours (but usually overnight) before staining to remove any compounds (e.g., carrier ampholytes, detergents) that might interfere with detection.
Specific staining methods for detection of posttranslational modifications (e.g., glycosylation, phosphorylation) are employed either directly in the 2DE gel, or after transfer (blotting) onto an immobilizing membrane. The blotted proteins can be probed with specific antibodies (e.g., against phosphotyrosine residues) or with lectins (against carbohydrate moieties).
9. Image analysis
The major steps of computer-aided image analysis include (1) data acquisition, (2) spot detection and quantitation, (3) pattern matching, and (4) database construction (Dowsey et al., 2003; see also Article 26, Image analysis, Volume 5).
Currently, several 2D image analysis software packages are commercially available. Programs have been continuously improved and enhanced over the years in terms of faster matching algorithms with lesser manual intervention, and with focus on automation and better integration of data from various sources. New 2D software packages have also emerged which offer completely new approaches to image analysis and novel algorithms for more reliable spot detection, quantitation, and matching. Several programs include options such as control of a spot cutting robot, automated import of protein identification results from mass spectrometry, superior annotation flexibility (e.g., protein identity, mass spectrum, intensity/ quantity, links to the Internet), and/or multichannel image merging of up to three different images to independent color channels for fast image comparison.
10. Protein identification from 2D gel spots
Mass spectrometry has become the technique of choice for identification of proteins from excised 2D gel spots as these methods are very sensitive, require small amounts of sample (femtomole to attomole concentrations) and have the capacity for high-sample throughput (Aebersold and Mann, 2003; see also Article 31, MS-based methods for identification of 2-DE-resolved proteins, Volume 5). Recent advances in mass spectrometry also allow the investigation of posttrans-lational modifications including phosphorylation and glycosylation (Mann and Jensen, 2003; Kalume et al., 2003) (see Article 61, Posttranslational modification of proteins, Volume 6, Article 62, Glycosylation, Volume 6, Article 63, Protein phosphorylation analysis by mass spectrometry, Volume 6, and Article 73, Protein phosphorylation analysis – a primer, Volume 6). Peptide mass fingerprinting (PMF) is typically the primary tool for protein identification (see Article 3, Tandem mass spectrometry database searching, Volume 5, Article 7, Time-of-flight mass spectrometry, Volume 5, Article 14, Sample preparation for MALDI and elec-trospray, Volume 5, Article 16, Improvement of sequence coverage in peptide mass fingerprinting, Volume 5, and Article 75, Mass spectrometry, Volume 6). This technique is based on the finding that a set of peptide masses obtained by MS analysis of a protein digest (usually trypsin) provides a characteristic mass fingerprint of that protein. The protein is then identified by comparison of the experimental mass fingerprint with theoretical peptide masses generated in silico using protein and nucleotide sequence databases. This approach proves very effective when trying to identify proteins from species whose genomes are completely sequenced, but is not so reliable for organisms whose genomes have not been completed.
If it proves impossible to identify a protein based on PMF alone, it is then essential to obtain amino acid sequence information. This is most readily accomplished with MALDI-MS with postsource decay (PSD) or chemical assisted fragmentation (CAF), or by using tandem mass spectrometry. MS/MS takes advantage of two-stage MS instruments, either MALDI-TOF-TOF-MS/MS or ESI-MS/MS triple quadrupole, ion-trap or Q-TOF machines (Article 8, Quadrupole mass analyzers: theoretical and practical considerations, Volume 5, Article 9, Quadrupole ion traps and a new era of evolution, Volume 5, and Article 10, Hybrid MS, Volume 5) to induce fragmentation of peptide bonds. One approach is to generate a short partial sequence or ‘tag’, which is used in combination with the mass of the intact parent peptide ion to provide significant additional information for the homol-ogy search. A second approach uses a database searching algorithm SEQUEST to match uninterpreted experimental MS/MS spectra with predicted fragment patterns generated in silico from sequences in protein and nucleotide databases (see Article 4, Interpreting tandem mass spectra of peptides, Volume 5).
11. 2D PAGE databases
Currently, enormous efforts are being undertaken to display and analyze with 2D PAGE the proteomes from a large number of organisms, ranging from organelles such as mitochondriae, nuclei or ribosomes to simple prokaroytes including Escherichia coli, Bacillus subtilis, Haemophilus influenzae, Mycobacterium tuberculosis, and Helicobacter pylori, to single-celled eukaryotes such as the yeast Saccharomyces cerevisiae (Figure 3), to multicellular organisms, for example, Caenorhabditis elegans, plants such as rice (Oryza sativa) or Arabidopsis thaliana, and mammalian cells and tissues including rat and human heart, mouse and human liver, mouse and human brain, different cancer cell lines, HeLa cells, human fibroplasts, human keratinocytes, rat and human serum and so on. Most of these and many other studies in progress are summarized at www.expasy.org/ch2d/2d-index.html (“WORLD-2DPAGE Index to 2D PAGE databases”). The Proteomics Standards Initiative (PSI) aims to define community standards for data representation in proteomics to facilitate data comparision, exchange, and verification (http://psidev.sourceforge.net/).
12. Concluding remarks
Although we have today a diversity of emerging proteomic platforms, there is still no generally applicable method that can replace 2DE in its ability to simultaneously separate and display several thousand proteins from complex samples such as microorganisms, cells, and tissues. 2DE using IPGs in the first dimension (IPG-Dalt) has proven to be extremely flexible with respect to the requirements of proteome analysis. Although by no means perfect, IPG-Dalt coupled with mass spectrometry remains the core technology for separating and identifying complex protein mixtures in proteomic projects at least for the foreseeable future.