1. Introduction
The mammalian cell is a complex system composed of many types of components ranging from simple inorganic molecules to complex macromolecular assemblies that form organelles. The complexity of mammalian cell arises from the large number of molecular components contained in the cell, the complex interactions between these cellular components, and the anisotropic spatial distribution of the components within the cell. The identity of the mammalian cell is defined by its genetic makeup. Gene expression programs allow for the transcription and translation of specified genes such that the expression of key proteins defines the phenotypic behavior of the cell. However, both the genetic code and the protein products are subject to changes: genetic codes can be altered by mutation caused by normal physiological or pathological conditions and protein molecules can be modified during their synthesis, for example, posttranslation lipid modifications steps, or after their synthesis, for example, biochemical reactions with other molecules such as phosphorylation. As a consequence, the system is composed of a very large number of molecules with different functional properties. For example, the total number of protein molecules in a liver cell has been estimated to be about 7.9 x 109; so, if there are about 10 000 different gene products in a liver cell, each protein can have on average nearly one million molecules (Lodish et al., 2000). Understanding how these molecules are distributed, regulated, and how they interact with one another is a challenge.
Advances in biotechnology, such as rapid sequencing of entire genomes, and in information technology, such as large-scale web-based databases, have led to the discovery of a substantial number of previously unknown genes and their protein products in viruses, bacteria, archaea, and eukaryotes. Many large databases have been constructed to store and analyze genetic sequences and protein sequences and structures, such as the nucleotide database GenBank of National Center of Biotechnology Information (NCBI online, 2004), the protein database SwissProt at Geneva (SwissProt online, 2004), and Stanford Human Genome Center (SHGC online, 2004).
In addition to the large number of cellular components, interactions between the components occur through a variety of mechanisms. These interactions often directly represent the functions of components within the cell system. For example, gene transcription is controlled by different transcriptional regulators that initiate, terminate, inhibit, to regulate transcriptional processes by interacting with each other and with specific genetic sequences. The interactions between components integrate distinct cellular components into a central regulatory signal network as well as functional machines within the cell. Interactions between the regulatory network and the machines result in the observed systems behavior (Figure 1). A defining aspect of cellular systems is its regulatory topology based on component interactions. The regulatory topology of the cell represents the dynamic structure of the system and determines system behavior, therefore directly reflecting the functionality of cell. Because of its importance, regulatory topology continues to be the target of intense research. Such research usually focuses on identifying the regulatory subcelllular maps of specific or common cellular signaling processes, such as G protein-coupled signaling pathways (Neves et al., 2002). The microarray technology developed in recent years (Spellman et al., 1998; Iyer et al., 1999) has been successfully applied to the measurements of large-scale genetic regulatory networks (Davidson, 2002), and even protein interaction networks (Nielsen et al., 2003). Various computational approaches have been developed to analyze the structure of these complex biological networks, such as the clustering analysis to identify coexpressed genes in genetic regulatory networks (Eisen et al., 1998), the statistical analysis to study the connectivity of metabolic biochemical networks (Ravasz, 2002) and probabilistic models to infer regulatory cellular networks from disparate biological data (Friedman, 2004) and identification of regulatory motifs (Milo et al., 2002).
A significant challenge in understanding intracellular systems is how a complex cellular system containing thousands of components, in response to internal or external signals, performs the desired physiological functions accurately in spite of occasional component malfunction or noise from adjacent processes. In other words, what are the mechanisms by which individual cellular components are integrated into a coherent and functional system? Intuitively, a straightforward approach to this challenge is to define an exhaustive anatomy of the cellular system by listing every component and every interaction. This is a good starting point, but it is not sufficient. It is like trying to assemble a complex mechanical machine from a number of individual parts. Although we know exactly what function each part performs and which other parts it can be assembled with, we do not know how to put all the parts together to build a machine that is able to perform its designed functions accurately, effectively, and robustly. What we need in order to build a working machine from its parts and interactions list is the design principles of the machine. More explicitly, design principles describe how to build the machine with required functionalities from the functions of the individual parts and the interactions between those parts. For example, which parts should be assembled together? What is the state of each part to generate specific required functions? How is each part controlled? How do we protect key parts? How do we resist systems perturbations during function? What is the working range of machine within which each part functions in an error-free manner? How do we recover from error? A well-defined design diagram would provide answers to these questions. In understanding the design principles of cells, we can take advantage of natural selection processes. The cell has been going through billions of years of evolution during which only those cells with optimized systems and functions survived. To satisfy rigorous requirements for survival, it is necessary to have functionality coupled with accuracy and robustness. One way to approach this issue is to assume that survivors must follow similar design principles as human-made machines. Compared with engineering, systems biological research is a reverse process (see Article 110, Reverse engineering gene regulatory networks, Volume 6). Nature has designed a complex cell system, and the task of systems biologist is to study and understand how this system works and thus identify the overall the design principles. Because the cell is a complex but coherent functional system, the approaches to study this system have to be “systems aware”, which means that we must analyze the cell from a systems perspective that can guide the design of experiments to explain the seemingly contradictory behavior of subsystems, search for hidden components, and predict unknown cellular behavior (see Article 107, Integrative approaches to biology in the twenty-first century, Volume 6). Such approaches are a major part of the emerging field of systems biology. This review covers two aspects of cell systems biology, dynamic machines, and design principles underlying the regulation of these machines.
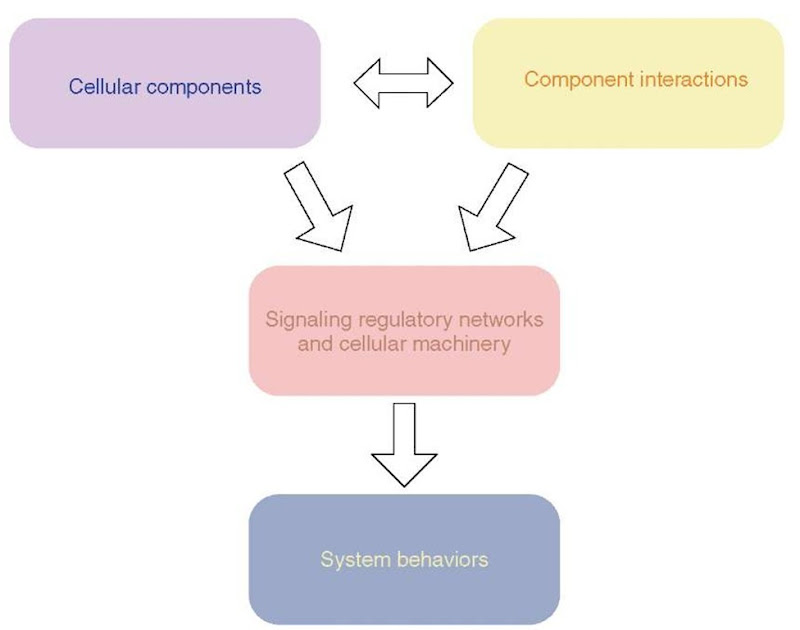
Figure 1 A schematic representation of a bottom-up approach to explain how systems level behavior is generated from components and interactions. The cell integrates cellular components and interactions into a functional systems by the means of regulatory signaling networks and cellular machines. Regulation of the functions of the cellular machines leads to the observed systems behavior
2. Dynamic machines
The phenotypic behaviors of cells arise from the function of subcellular machines as the vesicle release machinery or the electrical response machinery of excitable cells that are located in a spatially restricted manner. Such machines exhibit two key features: tight spatially restricted control of these machines and dynamic stability. Local machine functions result from the dynamic control of cellular machines by the central signaling network to perform time- and space-dependent functions. Dynamic stability allows the machine to maintain its functionality within the context of the function of multiple cellular machines operating simultaneously.
It is essential that, within the cell, a variety of cellular processes are scheduled to perform required functions at appropriate times. Although the cell contains a large number of components, each cellular process uses only a fraction of total components, which means that only the components necessary for current process are active while others are quiescent or engaged in other functions. For signaling networks, different signaling pathways are activated at different times (Jordan et al., 2000), and for genetic networks, different gene expressions are turned on at different times (Davidson et al., 2002). These dynamic behaviors enable the cell to assemble and disassemble local machinery to perform the functions required for a specific process. This feature distinguishes cellular machinery from human-engineered systems whose structure cannot be changed on demand. There are two known mechanisms by which these dynamic features can be achieved. One mechanism is the transient formation of local cellular domains solely by the interactions between machinery components (e.g., the spatial patterns formed by signaling reactions) (Eldar et al., 2002). The other mechanism is the localization of machinery components at cellular compartments (e.g., plasma membrane, cytoplasm, and nucleus) (Weng et al., 1999) and cellular scaffolds (e.g., actin cytoskeleton) (Medalia et al., 2002).
Once assembled, the local machinery performs its designated function for the specific cellular process. Such functions result from the components and their interactions determined by the machine’s regulatory topology. These local machineries can form higher-level cellular organelles such as the rough endoplasmic reticulum that contains both ribosomes and endoplasmic reticulum-bound posttranslational modifying enzymes required for the production of fully functional proteins. Therefore, the cell system can be always considered as a set of machines consisting of multiple components forming various levels of organization. The machines perform their functions by setting the components at preferred states through component interactions. These preferred states of components define the state of the whole machinery, and the state change of any components can affect the machinery state. For example, the fast growth of dendritic actin filaments is characterized by the high activities of machine components that regulate three processes: actin polymerization, actin depolymerization, and actin recycling. If the activity of any the components that regulates these processes changes, the overall growth rate will change (Pollard et al., 2000). Therefore, the dynamic behavior of cell system is governed by the state of the components that make up the cellular machines. Cells display both steady state and periodic dynamics. Steady dynamics is a status in which cellular machines operate at steady states, maintained by a constant level of activity with the central regulatory signal networks. Steady state dynamics may be observed both in cells functioning at basal levels as well as in cells that have undergone activity-induced state changes such as neurons that display the long-term potentiation and long-term depression of synaptic responses (Sheng and Kim, 2002). Cellular machines can also operate at multiple steady states. For example, the mitogen-activated protein kinase (MAPK) signaling pathway that connects extracellular growth factor signals with nuclear transcriptional machinery exhibits bistable states dependent on the signaling stimulation by growth factors, and interestingly, this bistable behavior can be altered to monostable behavior by other signals (Bhalla et al., 2002). Periodic dynamics is perhaps one of the most important behaviors of biological systems such as the population model of predator and prey in ecology and the electric oscillation in neuronal and cardiac cells. Particularly, periodic dynamics exists in the biochemical signaling networks that regulate a variety of cellular processes such as glucose metabolism, calcium oscillation, pulsatile hormone signaling, and gene transcription (Goldbeter, 1996). A very common biological periodicity is the circadian phenomenon in which biological systems can adapt themselves to the 24-h periodic variations of environment (Dunlap, 1999). An early detailed molecular model for circadian rhythms was based on a set of five biochemical kinetic equations that described the oscillations of the period protein of Drosophila (Goldbeter, 1995). However, the periodic oscillations of cellular machinery can go out of control and become chaotic when oscillation dynamics is highly sensitive to the initial condition of the system (Goldbeter etal., 2001). Owing to the complex nature of periodic phenomena, computational approaches are valuable tools to analyze such systems (Goldbeter, 2002).
3. Design principles
With the large number of components and interactions, combined with the dynamics of local machinery, cellular system can exhibit many uncharacterized complex dynamic behaviors besides the steady and periodic dynamics described above. Since such complexity greatly complicates the in vivo observations of cellular dynamics, it becomes increasingly difficult to explain these observations through intuitive speculation or brute-force exhaustive mathematical modeling. Instead, systems biologists can try to deal with such complexity by applying the concepts of system control from engineering theory (Zhou et al., 1995).
3.1. Modular regulation
Despite its complexity, the cell is a biochemical machine constructed from molecules, so it must follow all the physiochemical rules of nature. In order to generate the required biological functions and maintain life, cellular machines need to manage the tremendously large number of components and their interactions in an efficient way. This efficiency requires cellular machines to organize their components into distinct modules, each of which is responsible for specific functions. To control these modules, a well-designed intermodular regulatory system is present (Hartwell et al., 1999). Because modular regulation heavily determines the functionality of cellular machinery, many efforts, both experimentally and computationally, have been made to search for characteristic regulatory motifs that can explain various observations of the system. For example, autoregulatory motifs generate quick response to control input (Ren et al., 2000) and increase stabilility (McAdams and Arkin, 1997), chain regulatory motifs generate temporal sequential control (Simon et al., 2001), negative feedback motifs generate steady state dynamics (Buchman, 2002) and oscillatory dynamics (Goldbeter, 2002), positive feedback motifs generate bistability dynamics (Bhalla et al., 2002; Ferrell, 2002; Hasty et al., 2002), positive feedforward motifs generate quick response, large amplification, and high sensitivity to input signals (Goldbeter and Koshland, 1984; Lee et al., 2002; Shen-Orr et al., 2002), and multi-input motifs generate logical gate dynamics (Hasty et al., 2002). The design of these regulatory motifs for cellular machinery control share great similarities with engineering systems and highlight the physical origins of cellular systems.
3.2. Design of robust systems
Cells live in a world full of disturbances coming from environmental variation, intracellular and extracellular noise, and machinery malfunction. Systems within the cell must be able to maintain all the normal functionalities during these disturbances for the cell to survive, that is, the cell should display robust behavior. To achieve such robustness, cellular machines are designed with the following principles: (1) a relative intrinsic stability determined by the internal insensitivity of components themselves and their interactions to disturbance. Therefore, stability does not depend on machinery structure; (2) a dispersed regulatory structure that is determined by modular regulation. Both the physical and functional modularity of cellular machinery can prevent the malfunction or failure in one part of machinery from spreading to other parts and causing system-wide failures. Optimized modular regulatory structure can effectively stabilize the behavior of cellular machines against internal and external disturbances. For example, internal feedback regulation in the biochemical networks of bacterial chemotaxis makes the precise adaptation of bacteria to attractive chemical signals in environment very robust to the large variation of molecular concentrations and rate constants (Barkai and Leibler, 1997). Another example is the DNA robustness against damage by a feedback checking mechanism mediated by p53 protein. If DNA damage occurs, damage can be sensed by DNA-dependent protein kinases that can activate p53 protein. Active p53 protein then transactivates p21, which results in G1 arrest in cell cycle. This state is released when DNA is repaired. Thus, propagation of mutations due to DNA damage is limited. The design of cellular machines is a major mechanism for cellular machinery to attenuate intracellular noise to achieve various cellular functions (Rao et al., 2002); and (3) machinery redundancy, which introduces additional backups for important machinery components such that the failure of one component can be compensated by its substitution with another. Redundancy exists in both gene level and protein level. For example, at gene level, only about 256 out of 468 protein-coding genes are considered necessary and sufficient to sustain the existence of Mycoplasma genitalium bacterium (Mushegian and Koonin, 1996) and, at the protein level, chemokine proteins are redundant in both their production and their function (Mantovani, 1999).
Unless individual cell systems are intrinsically stable in terms of their components and component interactions, system robustness is often achieved by including extra machines that can serve the same overall function. Thus, redundancies could be at the level of regulatory motifs or in actual machine itself. In either case, the overall goal is to offset the influence of disturbance on cellular systems and maintain functional stability within limits. When cell systems contain a large number of components and perform various functions, substantial increases in machinery structure is required to maintain the robustness of the large system. Thus, the maintenance of system robustness relies on an essential feature of cellular systems: system complexity. Cell systems are thought to be complex due to numerous cellular components, a large number of component interactions, and the emergent complex system behaviors (Weng etal., 1999). However, the complexity of a cell system can be concealed by its robust adaptation to a variety of internal and external disturbances until the emergence of unexpected changes and failures. This type of approach can be better understood by comparison with the design of complex engineered products such as the Boeing 777 airplane and the allometric scaling chart of optimal cruise speed (Csete and Doyle, 2002). The design principles of biological systems share great similarity with those of complex engineering systems in terms of two aspects of system design: the design of machinery function and the design of functional robustness. Both types of design can significantly increase the complexity of the system. This is illustrated by considering some examples. The design of complex biorhythmicity and its effects on the behavior of metabolic and genetic networks (Goldbeter etal., 2001), or the systems with extreme robustness, such as robust control of airplane flight (Yeh, 1998), reveal a paradoxical systems property. System robustness achieved by the increased complexity of system machinery comes at the cost of system fragility, which represents unanticipated system vulnerability to malfunctions and disturbances. To achieve robustness, extra regulation of machinery modules are added to the system. Robust metabolic networks usually have scale-free type of connections between network nodes (Ravasz et al., 2002). This feature makes metabolic networks more robust to random node failure than randomly connected networks, but more fragile to system-wide failures when hub nodes fail (Buchman, 2002). Thus, system robustness and fragility are two intrinsically linked system properties originating from system complexity. Well-designed systems have to make deliberate choices to balance between robustness and fragility to achieve an optimal function (Carlson and Doyle, 2002; Csete and Doyle, 2002).
3.3. Initiation of cell spreading: A case study in signaling network regulation of the actin-based cell motility machine
Motility is an important physiological phenomenon.During development, the movement and migration of cells is very important for the proper development of the embryo. The ability to move in response to extracellular signals is a critical property of hematopoietic cells and the mounting of immune responses. Cell movement involves the actin cytoskeletal machinery as well as the tubulin-based cytoskeleton. Sheetz and colleagues have recently developed quantitative approaches to imaging the spreading of mammalian fibroblasts when they come in contact with extracellular matrix proteins such as fibronectin (Dubin-Thaler et al., 2004). We have started to develop a computational process to model this process in terms of components and interactions.
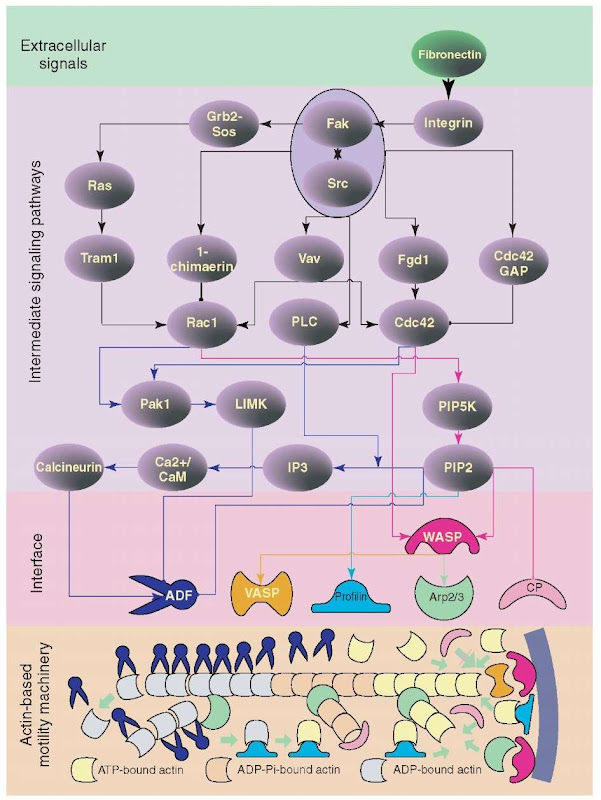
A simplified connections map that can be used to represent the regulated prohypbreakcess is shown in Figure 2. This system is made up of a regulatory signaling network and the actin cytoskeletal machinery. The regulatory signaling network senses extracellular motility signals, for example, fibronectin in this case, by integrin receptors on cell membrane and transduces the signal to the interface layer between the signaling machinery and the actin machinery. The interface layer consists of several actin-binding proteins capable of regulating the process of actin polymerization and depolymerization. Each protein in the interface layer receives signals from the respective pathway of upstream signaling pathway and in response to these signals regulates the corresponding aspect of downstream actin machinery. Then, the actin cytoskeletal machinery reorganizes the structure of the actin cytoskeleton during polymerization and depolymerization reactions, which results in growth of actin filaments close to the plasma membrane. This growth of filament pushes the plasma membrane, generating force required for the cell to spread.
The dynamics of this motility machinery is well characterized by the multiphase model observed for the fibroblast cell spreading in the experimental setup. This model describes the motility behavior of the fibroblast cell before, upon, and after it contacts the fibronectin substrate on glass slide. Three phases are observed: (1) the basal phase in which spherical fibroblast cell demonstrates random local membrane protrusion before it contacts fibronectin substrate; (2) the transitional phase in which fibroblast cell explores the spatial distribution of fibronectin substrate by a few trial contacts and then decides whether or not to spread; and (3) the spreading phase in which spherical fibroblast cell spreads over fibronectin substrate to form a flat disk. This multiphase motility of fibroblast cell is realized by the cooperation of the multiple aspects of the dynamics of actin cytoskeletal machinery, each of which is regulated by the corresponding signaling pathway of upstream signaling regulatory machinery. This modular design ensures the effective control of complex dynamics of actin machinery by extracellular signals and intracellular signaling machinery. Moreover, this motility machinery is designed to be both sensitive to extracellular signals as well as have an intrinsically robust performance capability. This complex behavior can be seen in the multiple phases involved in fibroblast spreading (Dubin-Thaler et al., 2004). The time needed for fibroblast cell to explore extracellular fibronectin in the transitional phase is dependent on the distribution concentration of fibronectin on glass slide: the larger this concentration is, the lesser is the time needed in this phase. This sensitivity enables fibroblast cell to move toward more concentrated extracellular signals. Thus, the trigger to move is directly controlled by extracellular signals. However, when fibroblast cell enters the spreading phase, spreading rate is independent of fibronectin concentration. This behavior suggests that once spreading is initiated, the spreading of fibroblast cell mainly depends on intracellular signaling regulation and control of the actin motility machinery and is insensitive to extracellular signal. Thus, the system exhibits a robust functional response above a threshold level of an extracellular signal.
Figure 2 A cellular motility system consisting of the regulatory signaling network and the actin-based motility machine. The biochemical components of actin-based and integrin-regulated motility of fibroblast cell is shown as a connections map. The system can be thought to consist of four layers: (1) the extracellular signals, here the extracellular motility protein fibronectin that induce fibroblast cell motility is shown. There are other extracellular signals such as growth factors and chemotactic peptides that can affect this process; (2) the layer of intermediate signaling pathways that interact to form a network, that contains the integrin receptor, GTPases, tyrosine, and Serine-Threonine kinases; (3) the interface layer formed by several actin-binding proteins, which receive signals from the intermediate signaling pathways and in response to these signals regulate the downstream actin cytoskeletal machine; and (4) the layer of actin-based motility machinery, consisting of actin filaments and monomers that by polymerization, branching, and depolymerization generates cell motility. This is a simplified connection map in which only key components are shown
4. Conclusions
Although the cell is a complex system, its overall design favors functional modularity and robustness. Functional modularity is achieved by an array of cellular machines that can operate in a coordinated fashion. These machines are highly regulated and a central signaling network coordinates the regulation in response to extracellular and intracellular signals. In order to achieve overall robustness, the system has developed extensive redundancies at both the level of regulation as well as functional machines. The inclusion of these redundant subsystems results in a highly complex system. The high complexity of cellular regulatory networks and machinery unavoidably brings fragility into the system. Successful survival represents the ability to achieve balance between the robust and fragile properties of the system through functional modularity.