Taking a very broad view, the “function” of marine mammals is to convert prey into offspring. Reproductive be*. havior is an important part of the process by which this is brought about and must serve to create a situation in which the young can safely be born and nurtured and one which facilitates mating with suitable partners. In long-lived animals, however, reproduction has to be linked to the process of gathering the resources for both reproductive effort and continued survival. Because many marine mammals do not feed where they reproduce, they must also locate breeding areas where reproduction and parental care can take place without compromising nutritional requirements. This article considers the basic problems that the animals must solve to reproduce and gives some illustrative examples of their behavior. We will take just such a broad, strategic view and look at reproductive behavior in a life history context and consider how animals balance their needs for resources and reproduction.
I. Basic Problems to Be Overcome
Although they spend most of their time in the water, seals give birth 011 land and most newborn pups require a period ashore before being able to cope with life at sea. The vulnerability of pinnipeds on land means that suitable breeding sites need to be isolated from potential predators, limiting the choice of suitable ones. Pinnipeds do not feed while ashore. The widely separated, patchy distribution of resources that typifies most marine ecosystems means that animals are often widely separated from one another while foraging and suitable breeding sites are often few and far between. This necessarily requires the use of stored reserves for periods of days to months. The geographical separation of feeding and breeding sites and the reliance on stored reserves are arguably the most important determinants of seal reproductive strategies and life history patterns.
Whales can give birth, nurse, and mate at sea, but conditions suitable for the birth of young may not be suitable for foraging so these two phases of their annual cycle can take place in widely separated geographical locations. Long migrations between breeding and foraging locations may still be necessary. While foraging, individuals might be widely separated from potential mates, creating difficulties for locating suitable partners. Little food may be available during the birthing and mating period, which therefore can require stored energy and materials for its success. Therefore, even though whales are not constrained to spend time ashore for breeding, in some cases, they face some of the same problems as pinnipeds.
On the whole, smaller cetaceans, including most odontocetes, opt for a different strategy. Foraging, parturition, and calf rearing overlap both spatially and temporarily. As a result, annual breeding migrations are absent and instead nursing females and calves appeal’ to be aided by associating with conspecifics.
Both seals and the larger whales must move to breeding areas and choose a suitable breeding site where they can safely give birth and protect and feed their young. They must choose a mate, copulate, and produce fertilized eggs. They must protect and feed their young and provide the resources and guidance needed for them to become nutritionally independent and give them a good chance of reaching maturity and recruiting into the breeding population. Then the adults must reestablish successful foraging patterns to provide resources for their own survival and reproductive success in the following year(s).
The marine habitat and the geographic and energetic constraints acting 011 marine mammals have shaped their life histories and reproductive behaviors to create some of the most dramatic and extreme (some might even say bizarre) reproductive patterns among mammals.
II. Importance of Size
Marine mammal groups contain some of the largest mammals in existence as well as possibly the largest animal to have ever existed. The size adopted by the various species is such an obvious characteristic that we often look past it to other features of the animals without considering its fundamental importance to behavior. However, size stands out as being of fundamental importance as to how these animals organize their reproductive behaviors. Because of the scaled relationship between body volume or mass (M) and metabolic rate (MR), where MR « M0’75, size has obvious implications for diving and foraging behavior. Larger species and individuals will require more prey each year, but they may be able to dive for longer and go longer without food and thus be able to contend with less predictable or widely distributed food distribution. It has equally fundamental implications for variations in reproductive behavior within and between species. Size in large part determines how long animals can fast during reproduction and how often they must leave their pups or the vicinity of potential mates for food. In general, bigger animals can maintain their presence on beaches for longer and can breed farther from food sources. Size also sets the relationship between the duration and the efficiency of lactation (energy used in the process divided by energy stored in the pup). It sets the weaning mass of offspring and the relative cost to the mother of achieving offspring of that mass; larger mothers can produce larger pups without putting themselves at risk. The metabolic overheads (i.e., the amount of energy required to support the metabolism of mother and pup) are relatively lower for larger animals in relation to the stored resources available and delivered to offspring. Size can affect the capability of animals (particularly males) to secure mates and, because of its influence on attendance patterns, can determine the sort of strategies used to gain access to females; larger males can maintain residence for longer on breeding sites. In the variety of strategies and behaviors used to accomplish reproduction and the factors that determine them, size matters.
Although seals and whales face common problems, the fact that whales do not come ashore to carry out any aspects of reproduction means that we have learned about their behavior in very different ways. Behavioral observations are largely confined to activities visible from the surface, and “hands-on” techniques are much more difficult to apply. Cetaceans also have greater opportunity for complex social interactions throughout the periods of mating and parental care because of the extended times occupied by these activities. The different methodologies used have also led to separation in the approaches used in the study of the two groups, resulting in emphasis placed on different aspects of behavior. It is therefore expedient to treat the two groups separately for much of the remainder of this discussion, even though we will be considering the same basic strategic goals. The walrus, manatees, dugongs, and the sea otter are dealt with briefly.
III. Otariids and Phocids
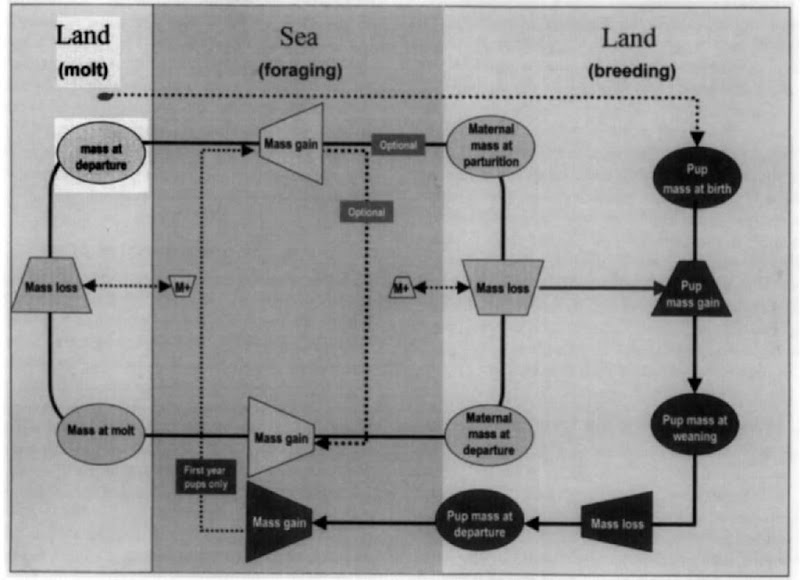
We consider the strategies of reproductive behavior within the simple life history model (Fig. 1) in which the animals mass or condition is viewed as the fundamental state variable that determines the constraints on reproductive success. It considers life history as an annual cycle of terrestrial and aquatic phases split among foraging, breeding, and molt (see Fig. 1 legend for details). Virtually all species can be fit into this conceptual framework, and most aspects of reproductive behavior and their links to foraging and molting can be incorporated within it, in terms of how they affect fecundity and offspring quality. As such, it provides a useful framework within which to describe the requirements of behavior.
Figure 1 A diagram of a model of female pinniped life history with mass (as a surrogate for condition) as a state variable determining reproductive success through fecundity and pup quality. Trapezoids represent mass gain and loss. Ovals denote mass at the start and end of lactation and molt. Dotted lines represent optional paths. Some species and/or individuals (particularly smaller phocids and otariid species and smaller individuals of some phocids) may top up body mass during breeding and molt by returning to the sea to feed. Pups usually bypass molt during their first year and do not breed until they have reached a certain critical mass or condition. Females may opt not to produce a pup in years when they are below a critical mass or condition. Pups that are larger or in better condition at weaning are likely to both be able to remain on the beach longer and depart in better condition. They have a better chance of surviving and to breed earlier. In this model, mass and condition determine the path taken and the resulting reproductive success rather than age perse (see Am-bom et al., 1997).
A. Transition from Foraging to Breeding: Locating a Suitable Place to Breed
This involves behavior that, at one extreme, may occur within a few meters, to the other extreme, movements that traverse the globe. Animals must choose a geographical area, a suitable site within that area such as a particular beach or ice flow, particular conditions within that site, and a position relative to other animals in the colony. Breeding site selection must be accomplished so that parturition can occur on time and suitable mates are also available.
This transition can be considered to occur as soon as animals switch from a period of net gain of resources to net expenditure of body stores to support travel to breeding sites. It is not likely to be a sharp boundary, as animals may encounter food during their trip, but a reduction in resource acquisition is likely because animals are likely to leave prime foraging areas to make their way back to breeding sites. The critical issue is that animals arrive at the breeding site in sufficiently good condition to support the onset of breeding. In the case of many otariids, this means that females must have sufficient reserves to sustain themselves and their pups until the mother’s first successful foraging bout. Condition in males will in part determine how long they can remain defending their access to females. In the case of the larger phocids, animals must have sufficient body condition to support the entire breeding effort. Smaller phocids or those with easy access to food may supplement stored reserves with foraging. It could be argued that the expenditure during this phase should be added to those of reproduction, but we know of no studies that have attempted to do this. Navigational skills and previous experience of suitable sites allow the minimum time and energy to be expended.
1. Large-Scale Movements: Choosing a Geographical Location Many species have been shown to have the navigational skills to return to previously used breeding sites from great distances, but the methods they use to accomplish this remain largely unknown. Both southern (Mirounga leonina) and northern (M. angustirostris) elephant seals have been tracked making directed trips of 1000-3000 km between breeding and foraging locations, arriving on the same beaches they used for breeding the previous season. Their great size (males may weigh over 3000 kg and females average 500 kg at parturition) is important in making such trips energetically feasible. Many other larger species are similarly capable. Smaller species may need a supply of food on route or feed closer to where they breed as the reproductive season approaches. It seems likely that the evolution of large body size in pinnipeds may have had much to do with enabling the uncoupling of geographical locations of feeding from those of breeding (for a contrary view, see Boyd, 1998).
2. The Local Scale: Choosing a Breeding Site within a Locality At the local scale, animals tend to breed where there are other seals present. This aggregative sociality is a key feature of pinniped behavior, although it is modified by the animals’ state. The first animals to breed in a season tend to be the older, more experienced animals and their presence ashore encourages others to use the colony. This reinforcement means that the same rookeries tend to be used over a long term. It is possible to envisage at least two ways that new colonies may form. In a growing population, when space at an established colony has become limiting, pregnant females arriving to breed may be forced to move elsewhere if the available habitat is being used. In this scenario, the new colony should be reasonably close to the original one. Younger, primiparous females that breed later in the season may be forced to use an otherwise unoccupied location, and once there, others join them. Main criteria listed for breeding habitats are isolation (protection from land-based predators) and access to resources nearby. Few seals stray far from the sea. Otariids require a plentiful supply of food within reach of the breeding location, otherwise the breeding attempt may fail, but most phocids are functionally divorced from foraging requirements at breeding time by their ability to store resources, principally as blubber. Thus phocids may use breeding locations far removed from their foraging grounds [e.g., gray seals (Halichoerus grypus), northern and southern elephant seals]. Harbor seals represent an intermediate breeding and feeding strategy where mothers supplement their stored reserves with food acquired during foraging trips late in the lactation period.
3. Choosing a Position within a Site: The Individual Scale The local topography of the breeding location plays an important role in determining the particular location where pups are born. An almost flat and featureless surface with unlimited access to the sea, such as a sandbank, offers the simplest case in which animals have little to choose except their distance from the sea. Where such a location also has additional resources such as pools of water, these may act as foci for breeding animals. However, seals breeding on many islands or beaches typically arrive at the breeding area through specific access routes. Restricted access produces a radiated pattern of colonization, but also creates thoroughfares where there is continual traffic as animals arrive and leave the colony. Pup mortality in these locations can be substantial.
The degree of topographic variation on a breeding colony at a scale relevant to seals is a primary determinant of their distribution within a site. In fact, the degree of topographical variation on the breeding colony probably also defines the scale of site fidelity shown by gray seals at two Scottish colonies and may explain why fidelity is less apparent at relatively flat, open locations such as Sable Island (see later; Pomeroy et al., 2000).
Conservation or management considerations often require information on how animals may use available habitat, particularly where multiple use or potential conflicts occur. Habitat classification within a fine-scale geographic information system has been used to identify suitable breeding areas for gray seals, which was used to make a successful prediction of expansion of breeding areas at the Isle of May colony during the 1990s (Twiss et al., 2000, 2001). One of the main benefits of having such a detailed digital terrain model is that animals can be mapped accurately and frequently in the field, allowing examination of temporal changes in the distribution of animals. One of the products of such an approach has been the identification of the importance of pools of water on the colony for seal distribution and behavior (Twiss et al., 2000).
4. Assessment of Breeding Locations and Site Choice Seals that breed on land come ashore for a variable period before parturition occurs. For southern elephant seals, this averages 4.5 days, whereas for gray seals the average time ashore before parturition is 2-3 days, although some animals are present at breeding locations for at least a month prior to parturition. This prepartum period appears to involve some assessment of the breeding location. Female gray seals emerge from the water, looking around intently and sniffing continuously, before making tentative moves inshore. Pregnant females collect together in groups near access points where they remain inactive, and any disturbance is likely to make them return to the sea. In some cases, final selection of the pupping site occurs immediately prior to parturition when females move inland; during this movement they can be seen sniffing the ground. Pomeroy et al. (1994) found many cases of females returning to the sea before they came ashore again to pup, sometimes in a different location to that chosen originally. Options for changing the pupping site are often limited to the prepartum period, as in most species, once the pup is born, it is not easy for mother and pup to change location together.
Once a seal has chosen a breeding site, it tends to be used again and again. This breeding site fidelity is shown by Wed-dell seals (Leptonychotes weddellii), gray seals (see later), northern elephant seals, and Antarctic fur seals (Arctocephalus gazella) and is probably widespread. Scottish gray seals are faithful to their previous pupping sites. Females return to pup within an average distance of 55 m on North Rona and 24 m on the Isle of May from their previous breeding sites (Pomeroy et al., 1994, 2000). Males that return also show veiy similar spatial fidelity at both colonies (Twiss et al., 1994; Pomeroy et al, 2000).
Some seal species display philopatry, i.e., they return to breed at the location where they were born, e.g., fur seals (Gentry, 1997) and southern elephant seals. Gray seals also display philopatry, sometimes with remarkable accuracy (Pomeroy et al., 2000).
B. Investing in Young after Birth
Parental care in pinnipeds is the exclusive domain of the mother in all but one species (see later for single exception). Males take no part in the rearing process; the only part they play in breeding is to contribute sperm during mating. In fact, the process of mating is not without risk to current offspring. Pups may become separated from their mothers or be the subject of aggressive behavior from males or females at this time, as well as running the risk of being crushed by males. Therefore, maternal care does not consist simply of feeding but includes all behaviors associated with the pup’s welfare, such as maintenance of contact, vigilance, and defense against potential aggressors. A mother has finite resources available to service each breeding attempt and, once the pup is born, she has ultimate control over the feeding schedule and its duration. Mothers must gauge their reproductive effort according to the resources they have available, to do enough for the pup to have a good chance of survival without prejudicing the mother’s survival or future breeding chances. The costs for mothers that expend too heavily in 1 vear are reduced fecundity and lower breeding success in the next year (Pomeroy et al, 1999; Trillmich, 1996). Consequently, the fundamental maternal trade-off is one of efficiency: supplying resources to the offspring at a low or acceptable expense. The single most important influence of the efficiency of the process is the “physiological time” it takes to accomplish it (Anderson and Fedak, 1987). For most phocids at least, maternal maintenance during the lactation fast must occur in parallel with the demands of feeding the pup. The shorter this time, the smaller the fraction of maternal resources that are lost as heat (generated by the combined metabolism of mother and pup) and the greater the fraction that can appear as pup growth or remain as maternal condition.
The conflict between pups’ demands for resources and the requirement ol mothers to limit expenditure to that which does not incur a threat to themselves is exemplified by a study of southern elephant seal pups fed as twins. In this case, mothers did not expend resources beyond the level expected for a single pup, so that the cutoff point in this case was fixed by the mother (Arnbom et al, 1997).
1. Maintaining Contact with Pups Seal breeding colonies are typically sensory-rich environments. Many animals are crowded into a restricted area, with the associated sights, smells, sounds, and actions associated with such a situation. A mother must maintain contact with her pup because, in general, neighbors react aggressively to foreign pups and pups that move away from their mothers may be injured or lose contact with their mothers and starve. At birth, a mother immediately sniffs and interacts with the neonate. By the time the first feed has been completed, mothers have established a bond with their pup that becomes progressively stronger as lactation proceeds. In most species, pups vocalize almost as soon as they are born, with mothers displaying a varying degree of competence in discriminating their own pup’s call (fur seals, reviewed in Gentry, 1997). In gray seals which commute from the breeding beach to the water during lactation, a returning mother looks, sniffs, and (presumably) listens when she approaches her pupping site. Often several pups are inspected before one is fed. Reunions involve the approach by the mother, sniffing and flip-pering of the pup, and finally presenting the nipple to feed. Other pups trying to feed at this time are often dissuaded by aggression, but may also be excluded by the mother turning away. Some gray seal mothers (particularly at the expanding colony on the Isle of May, Scotland) are poor discriminators and feed any pups that approach them. Not surprisingly, these mothers rarely wean large offspring (see earlier discussion). Otariid mothers leave their pups unattended for several days while they forage for food and must recognize their offspring on their return. The primary mechanisms allowing this to occur successfully are smell and sound recognition.
2. Providing Protection Until the pup is born, many species of seals are tolerant of other conspecifics so that, for example, large groups of pregnant female gray seals may lie very close to each other, often touching. As soon as the pup is born this tolerance disappears and the mother becomes fiercely protective of the pup, defending a radius (typically 1.5 body lengths) around it. Any intruders into this space experience an escalating aggressive response, beginning with threats, approaches, then vocalizations, flippering, lunges, and, finally at the most extreme, contact involving biting and flippering. Tolerance of conspecifics varies between mothers, but it is not yet known whether this reflects some form of kinship recognition, familiarity based on nonrelated associations, or simply individual variation in response.
There is a single instance of possible paternal care in pinnipeds. where male Galapagos sea lions mob sharks around colonies (reviewed in Trillmich, 1996).
3. Lactation and Weaning The process of lactation is demanding for mothers. Most phocid species that fast during lactation lose 30-40% of their postpartum mass, much of it blubber, producing the highest-fat milk known in the animal world (up to 60% lipid) in the process. In most phocids the lactation period is short but intense (hooded seals, 4 days, pup growth rate 6.0 kg/day: gray seals, 18 days, pup growth rate 2.0 kg/day; southern elephant seals, 23 days, pup growth rate 4.2 kg/day). Such growth rates can only be achieved by having energy-dense milk, frequent feeds (every 4-5 hr in gray seals), and efficient conversion of maternal resources by the offspring. The concentrated milk also conserves water, which may be of short supply. Otariids have fewer absolute reserves available, although these may be relatively similar to those of phocids, and sustain their energy requirements by foraging throughout their extended but less intense lactation periods. This means that otariid pups receive feeds at intervals several days apart.
Weaning is abrupt in most phocids. as females depart from the rookery to return to the sea. leaving the pups on the beach. In most cases, mating has already occurred, and indeed observation of a successful mating is a good indication of a female’s imminent departure. However, there is considerable individual variation in the time that mothers spend with pups after mating; some may remain for several days before returning to the sea. In many otariid species, a long lactation period allows offspring to develop swimming, diving, and foraging while having the option of maternal milk as a food source. As a result, otariid mothers may have a much more prolonged weaning process, as offspring may still be widi their mothers in the second year after birth.
C. Locating and Selecting a Mate
Reproduction is the single most important action that individuals of any species carry out in their lifetimes. As such, mate choice is an important consideration. Circumstances dictate the degree of choice likely in that the distribution of females at breeding time controls the mating patterns seen. For example. solitary hooded seal (Cystophora cristata) mothers on fast ice are unlikely to have many options in available mates and may simply mate with the male that has waited persistently beside her until she entered estrus. However, it seems likely that the successful male may have had to defend this position in encounters with others attempting to gain this opportunity. In this situation, males give the appearance of being monogamous. In contrast, large aggregations of female southern elephant seals make it possible for males to attempt to control access for mating, with the result that extreme polygyny occurs. Males in this situation compete vigorously among themselves, as die potential breeding rewards for successful males can be substantial. However, the priorities of each sex are rarely symmetrical. Female elephant seals may be considered to have exercised mate choice just after they arrive at the breeding beach. The 4.5 days spent ashore prior to parturition offer females an opportunity to assess the stability and safety of the harems they enter and the qualities of the guarding males. If pregnant females are disturbed during this preparturition period, they often change locations.
1. Female Mating Behavior Female seals are not receptive to males until they enter estrus. In gray seals, this occurs around day 15 of the average 18-day lactation period. Males that attempt to mate before the female is receptive receive a robust and clear message from the female indicating her unwillingness. Initially, a female will threaten males that approach and her subsequent vocalizations at a persistent male can alert surrounding females to his presence. Neighboring females may join in this threat display to dissuade the male, although in a very few cases, fights between male and female may develop. Because of the sexual dimorphism common to most polygynous mammals, males tend to be favored in such encounters. Females dissuade males using the same repertoire of aggressive behaviors as described earlier, but with the additional consideration diat males attempting copulation are likely to have tried to mount the female. In this situation, the female’s mobility and lack of cooperation, together with the aggressive display, are usually enough to make the male withdraw. Experienced males rarely attempt more than a preliminary investigation into the female status and seem particularly adept at gauging a female’s receptivity.
The ability of females to resist premature advances is perhaps at its most dramatic in elephant seals, where males can be more than 10 times the mass of the females with which they mate. Even here, in a species in which males are not known for their gentility, females can repel unwanted advances. A male holding a harem frequently accesses the receptivity of females by “heading” them. i.e.. he approaches and rests his head on the neck of candidate females. If the females are not receptive, they move their hind flippers rapidly from side to side in a swimming motion, slapping the side of the testing male. Most males take heed and move quickly on to test other females.
It is not clear exactly how estrus is signaled in most species. While the general behavioral indications are simply that a female’s initial aggressive response to a mating attempt declines to acceptance and passivity, it is not clear what cues a male uses to judge the situation. Olfaction is probably important as males can be seen sniffing during their approach around females (Gentry, 1997). A successful mating may also signal to other males that a female is receptive. Some females approach males and apparently solicit their attention.
Mate choice may range from having a single candidate, and therefore a passive default, or be an active process involving the assessment of, or competition between, a number of candidates. Competition may even be among sperm, in cases where multiple matings occur (e.g., gray seals, elephant seals, some fur seals). The most comprehensive studies so far come from gray seals and elephant seals. Females that occupy prime sites on gray seal breeding colonies tend to have dominant males nearby (Pomeroy et al, 1994, 2000; Twiss et al, 1994) and most mate with the dominant male. However, the number of pups they produce that are fathered by that male does not reflect the males behavioral dominance or his mating success. In addition, evidence for genetically dissimilar partners has been put forward (Worthington Wilmer et al., 2000). The reasons for this are not yet clear, but may lie in different attendance patterns of individual females at breeding colonies. There is some anecdotal and circumstantial evidence of mate choice in gray seals. At North Rona, where approximately 1200 pups are born each year, the father of a pup born to female J8 in 1986 was seen next to her in 1993, but both were at the other end of the island from where they had been in 1986. The pup born to J8 the following year was indeed fathered by the male seen with her in 1993. A more intriguing occurrence was first observed in 1997, when a known female left her peripheral pupping site to move about 80 m to the center of the colony where she was mated by a dominant male. She then returned to her pup and the attentions of a peripheral male at her pupping site. Females have been seen initiating copulations, but males initiate most.
2. Male Mating Behavior A males reproductive success is dependent on the number of offspring he manages to sire and how many of those eventually manage to reproduce as adults. The first part of this requires successful matings, and to achieve these, males must be able to take up a place among breeding females, avoid or outcompete other males, and gain a successful copulation. Males employ a variety of strategies to achieve success (see later). The second part of his reproductive success is less straightforward, as it is possible to achieve many matings without producing any surviving offspring, let alone grandoffspring.
The first prerequisite is simply to be around breeding females. Males must coordinate their efforts with the availability of receptive females. One of the most effective ways of gaining success for males is to spend a long time on the breeding colony, but this is costly, both in energetic terms, because males usually fast, and in potential injuries inflicted by competing males (Twiss et al, 1998). For these reasons, a large size tends to correlate with male success so that the largest males tend to have advantages of increased energy reserves and of greater competitive abilities.
As discussed previously, the potential for polygamy in these animals depends on the distribution of females. Although the terms monogamy and polygamy usually apply to mating patterns of species, they may be applied to the tactics that individuals employ either throughout or during phases of their lifetimes. However, without complete knowledge of the reproductive histories of individual animals, it is difficult to make generalizations. Evidence from genetic studies can provide useful insights in these areas. In general, the evidence to date from genetics supports the general observational conclusions on mating patterns, e.g., in southern elephant seals and gray seals, although some queries have been raised. One such is the failure of apparently dominant males to account for as many paternities as predicted. Male reproductive longevities are as important as their within-season success. Long-lived, repro-ductively active males may accrue a greater success than live-fast, die-young males whose activities are conspicuous.
As with many mammals, the risks inherent in engaging in fights over breeding have led to a formalized ritual of aggressive displays in many pinniped species. Dominance hierarchies are common so that disputes lead to fewer actual fights than might be expected. Fights occur, but usually between closely matched opponents where the preliminary assessments could not determine a clear outcome (Arnbom et al, 1997; Twiss et al., 1998). In gray seals, males attempt to control access to groups of females by threatening intruders with open mouth displays, hisses, and vocalizations. Intruders are chased away, but serious challengers may produce fights, which can last up to an hour and leave either or both combatants seriously injured. It is common for losers of such fights to disappear from the breeding colony.
Given the high cost of engaging in the mainstream competition for mating opportunities, it is not surprising that alternatives strategies exist. Younger, less experienced males are seen around the periphery of breeding colonies and may acquire experience gradually. Some males employ a cryptic tactic, using their similarity to females to gain a position among females, making the most of their opportunities when the dominant male is engaged elsewhere. It is becoming evident for some species that aquatic mating may occur to a greater extent than had been suspected and that the phenotypic qualities that are successful on land may not necessarily be the same for aquati-cally mating males.
D. Mating
In most observable species, males usually initiate copulations. Males often act to immobilize the female in some way by holding or biting the back of the neck. On land, the male’s weight applied via his body or flippers can help position the female. In the water, because animals are near neutrally buoyant, the male’s weight is less important in restraint. In gray seals breeding on land, a male attempts to mount the female by maneuvering alongside and then throwing his head and shoulders over the female’s back. Her response is almost always aggressive, but a female in estrus will accept the male’s advances if he persists and manages to grasp the skin of her neck in his jaws. This act is the single best predictor of a female’s acquiescence. At the same time, the male tries to achieve intromission by repeated pelvic thrusts, while the female either cooperates by lying still or resists by moving her rear as much as possible. Gray seals also mate underwater. There too, males grasp the females by the back of the neck in their jaws. Because the male cannot restrain the female as easily, she has greater opportunity to avoid the mating. Obviously, both must breathe and both move together to the surface when necessary. It is not clear how the need to breathe is communicated to the other partner, but cooperation is evident. In both situations, when a successful mating is achieved, the pair remains relatively motionless, for anything as brief as 5 min or as long as 40 inin (gray seals; Twiss et al, 1998). The function of such long copulations is not known, as males indulging in long copulations are leaving other females unguarded. It is thought that ejaculation occurs toward the end of the copulatory period; certainly females have been observed to have rhythmic contractions of the lower abdomen in the later stages of copulations. Remating of a female may take place soon after a copulation, either by the same or a different male.
E. The Transition to Foraging
1. Postiveaning Behavior of Mothers The mothers of most otariids leave their pups repeatedly to feed and gather the resources to support continued lactation. For them, weaning does not therefore represent a dramatic change in behavior. They simply fail to return. Some species, such as the Galapagos fur seal (Arctocephalus galapogocnsis) may give birth to the subsequent pup before the prior pup is weaned, but this pattern is unusual. For most, animals may shift to nonbreeding haul-out locations and engage in longer and more distant foraging trips. For phocid mothers, however, weaning occasions an abrupt change in behavior. Typically, soon after mating occurs one or a few times, mothers abandon their pups, leave their position in the colony, and quickly enter the sea. In some species (particularly among otariids), animals may be seen traveling away from breeding sites in groups, but in many phocids, departure appears solitary. Pups are normally left behind. The “decision” to leave is a critical one in relation to the state of body energy reserves. Good foraging areas may be distant from breeding locations and have changed in position and value while animals were breeding. Mothers must have sufficient stored reserves to enable them to reach these without putting themselves at unacceptable risk.
2. Postiveaning Behavior of Pups Weaning prompts dramatic changes in behavior for pups as well. Once pups are weaned, they no longer have the protection of their mothers. They no longer nurse and begin to fast for a time before the transition to nutritional independence. Otariid pups undergo what may be thought of as temporary weaning, when their mothers depart to sea to feed in between bouts of lactation. They have the experience of being left unattended prior to true weaning and show some of the behaviors of weaned pups early in development. In either case, pups typically move to areas where they can avoid contact with adults and may often congregate in large groups. In many cases, this movement is stimulated by aggressive encounters with other mothers and adult males within the colony. In elephant seals, mothers leave the weaned pup behind in the harem, possibly at a central location within it. Other mothers will act aggressively to the approach of pups other than their own, which tends to move unattended pups around, with a net movement to the periphery of the harein. Within a day of weaning, pups are usually out of the harems and then move around the beaches in an apparently undirected way. When they encounter other pups, they tend to remain with them. The end result of this mobility is that large numbers of pups end up in “creches” at places on beaches where no harems are present.
Phocid pups often remain in such groups, associated with breeding sites, for periods from days to months after weaning. The function of the time spent in these “postweaning fasts” is not understood, but is thought to involve a period of physiological, behavioral, and/or social development. Pups may interact with one another, exhibiting some variants of adult behavior. For example, male elephant seal pups often engage in mock fights that involve the rearing up and head strikes seen in battles between breeding males. Pups of both sexes tend to move into shallow inshore water or freshwater ponds during the night and swim and dive. Dive depths as great as 271 m have been observed in pups from Macquarie Island during this time (Hindell et al, 1999), but in general dives are short and shallow. An increasing fraction of the day is spent in the water until departure on the first foraging trip, typically after about 30-45 days and after about 35% of the mass attained by weaning has been lost.
For pups too, the decision on when to leave is potentially difficult and critical. Phocid pups have no prior, independent experience of foraging locations. They tend to leave as individuals, not forming into groups to avoid predators, and in any case, any other pups departing after weaning are similarly naive. The way they choose to locate foraging sites and the cues they use to help them remain largely unknown. Even otariids and phocids with unusually precocious young do not appear to use the opportunity for mothers to lead pups to food. Walruses (Odobenus rosmarus) seem to be the only exception to this. Pups travel with mothers prior to weaning and nurse at sea, giving them the opportunity to get geographical information on where to feed. It seems likely that if mothers could direct pups to food, they could obviate the need for a fraction of the material resources given to pups with this information. It seems surprising that this occurs rarely.
IV. Walruses, Sirenians, Sea Otters, and Polar Bears
While facing the same fundamental problems as seals in accomplishing successful reproduction, these groups show some unique variations in reproductive behavior and other life history features. Detailed accounts can be found in Fay (1982) for walruses and Riedinan and Estes (1990) for sea otters (Enhy-dra lutris). This section discusses only those features peculiar to these groups, emphasizing the unusual features of reproductive behavior.
A. Walruses
Walruses share many features of their breeding behavior with phocid and otariid seals but differ in that much of the important behavior takes place underwater. Like many species of seals, they are polygynous. Males display and interact aggressively to gain access to females, but unlike some phocid and otariid species, this activity tends to take place exclusively in the water, where mating also occurs. Both sexes are often highly gregarious when hauled out on ice or land, and animals are often seen in closely packed groups. While the activities performed out of water may have important physiological and social functions, with the important exception of parturition, they do not seem to relate directly to reproduction. Pups are born on ice or land but unlike seals, nurse at sea. Nursing is often seen when a mother orients herself vertically with her head above water while the pup nurses upside down, hindflippers at the surface and head down at the nipples. Walruses are the only pinniped group where offspring are known to accompany mothers during maternal foraging and this is made possibly by the ability to feed pups at sea. Because walrus offspring move about with their mothers when they feed, young have the opportunity to learn about foraging locations and techniques Irom their mothers, which probably has important implications for the demands placed on mothers by lactation.
B. Sirenians
The manatees (Trichechus spp.) and dugong (Dugong dugon). are unique in being the only group of mammalian marine herbivores. This lifestyle has led to unusual distribution patterns as well as unusual breeding and social behavior. They are not colonial breeders. Individuals of both sexes move in response to the availability of resources such as food and fresh water but they do not to move in herds while foraging. The only apparent long-term social link seems to be between mothers and calves (see later). Florida manatees (T. manatus latirostris) living at the northern edge of their range congregate in large groups around warm water sources, such as power plant effluents and warm springs. Although they seem dependent on warm water sites at times of exceptionally cold weather, this opportunistic proximity is not utilized to bring the sexes together for mating. Males and females can range widely at other times. Locating mates seems to be the result of chance encounters between males and estrous females. Little is known about how males locate estrous females, but the increased mobility of estrous females may increase chances of encounters. When a female comes into estrus (lasting up to 3-4 weeks), “mating herds” of hopeful males surround her. The generally quiet and gentle appearance of the species is belied at this time with aggressive behavior between males trying to secure mating opportunities. During estrus, females may mate with several males. Calves are born underwater and nurse there, accompanying their mothers for 1-4 years. Contact between mothers and young is maintained in part acoustically. The extended period of contact between mother and calves while animals forage offers ample opportunity for information to be passed from a mother to her young. This is in marked contrast to the situation described earlier for most pinnipeds and probably has played a role in setting up what has been termed “learned traditional patterns of distribution.” The seasonal aggregations of Florida manatees at cold weather refuges such as the springs and power plants mentioned earlier often contain individuals from several generations. Small groups often also congregate briefly at so-called “rendezvous sites” (often places where watercourses intersect) where animals may interact, rubbing and swimming around one another. The social role of these interactions is not understood fully.
C. Sea Otters
Sea otters probably arose from a different evolutionary lineage than the groups discussed earlier from whom they show some important differences in reproductive behavior. Like sirenians, they are not colonial breeders. Although they have a polygynous breeding organization, males occupy and defend a territory that is used for foraging and reproduction. Even though they seem less specialized for a marine existence than seals, sea otters spend virtually all their time at sea, foraging, mating, giving birth, and rearing their young in the shallow coastal zone. However, they are limited to relatively short, shallow dives and spend a greater proportion of time at the water surface than most seals and whales. Most of their reproductive behaviors occur there. Females tend to have partially overlapping home ranges along the coastal zone. Adult males have ranges that overlap with those of one or more females but not of other males. Higher quality male territories (e.g., those rich in food or other resources) tend to overlap with those of more females. Males defend their ranges, but this is often accomplished without obvious aggression, possibly via cues that allow individual recognition and the use of knowledge of past encounters to settle disputes. Males may leave their territories from time to time and may compete elsewhere with other males for access to females.
Mating takes place at the water surface and can involve precopulatory touching and playing. During copulation, males may grasp females by the muzzle while the pair tumble about vigorously. The grip of the males often causes injury and permanent scarring. Pups are born and nurse at sea and may spend 5 months or more with their mothers. Information exchange between mothers and pups is likely to play a role in determining the survival of the young. Pups are often provided with prey by their mothers and may show preference for the same prey types. Sea otters are well known as “tool users” (using hard objects to break up prey) and these skills may also be passed from mothers to pups. Mothers and pups can be quite vocal, calling to each other at the surface using a variety of sounds. Again, males play no role in the care of young.
We are left with the intriguing question: if the ability to give birth at sea, nurse in the water, and lead pups to food is possible in the walrus, sirenians, and sea otter, why do these patterns not occur more often in the “mainstream” pinnipeds? It is true that most extant pinnipeds produce young that are not equipped with the insulation to enter cold water directly from the womb, but in a few species (e.g., harbor seals), pups do enter the water within hours of birth. Aquatic parturition and nursing therefore seem to be possible evolutionary options for the pinnipeds as they are for these others groups and, as discussed later, cetaceans.
D. Polar Bears
Polar bears (Ursus rnaritimus) are considered marine mammals because they range widely over sea ice foraging on seals and often enter the sea to swim relatively short distances to and from ice floes and land. They tend to fast during the summer months when sea ice is absent. Their reproductive patterns are similar to those of brown bears (U. arctos). After a period of delayed implantation and a relatively short 3- to 4-month gestation period, the small (approximately 0.5 kg), altricial young are born in dens on land near shore or occasionally on stable sea ice. At this stage both mother and young exist on the mother’s stored reserves, acquired from the previous winter’s foraging out on the frozen sea. Cubs remain in the den with the mother until they grow to about 10 kg and then may spend some additional time in the den area before mother and cub move away onto the sea ice to look for food. Cubs spend around 2 years with their mother, presumably learning and gaining hunting experience, so there is ample opportunity for information to be transferred from mother to cub.
Male bears will kill and eat cubs if their mother does not defend them, and mothers will not associate with males or mate until cubs are independent. It is not clear how males find and recognize receptive females, although olfaction probably plays an important role.
V. Cetaceans
Unlike pinnipeds, cetaceans have evolved a behavioral and anatomical suite of adaptations allowing them to mate, give birth, suckle, and nurture their young entirely in water. Freed of the spatial and temporal constraints imposed by reliance on land or ice to breed, cetaceans have developed a wide diversity of social systems and life history strategies quite unlike those of the pinnipeds. While some cetaceans, principally the mysticetes, compartmentalize breeding to a temporally and spatially discrete component in their lives, the majority breed and acquire resources simultaneously. Further, consecutive breeding attempts themselves may be superimposed upon each other, with females concurrently rearing calves from different breeding attempts and even contributing directly to the survival of their offspring’s own offspring.
Having no need of land to reproduce has assuredly led to the success and ubiquitous nature of the cetaceans, but for the same reason has also severely hampered our abilities to understand them. At the most basic level, discrete acts, such as copulation and birth in most species, have never been observed, let alone quantified, while comparing the success of different tactics employed by individuals within populations is simply impossible as yet. What is known is pieced together from anatomical studies, whaling operations, live captures, individual identification, genetic analyses, and interspecies comparisons. From these fragments, it is clear that the cetaceans have much to teach us about the ecological determinants of reproductive and social behavior and even offer the potential to broaden our understanding of mammalian reproductive behavior as a whole.
A. Seasonality of Reproduction
For the majority of cetaceans, reproduction has a seasonal component. For mysticetes (with the possible exception of Bryde’s whales, Balaenoptera edeni/brydei), breeding occurs as a discrete phase of each year with other aspects, principally feeding, often being either reduced or halted entirely. The best-known species shuttle on an annual basis between productive feeding regions and areas associated with parturition, early nursing, courtship, and mating. The reproductive behavior of gray whales (Eschrichtius robustus) is a prime example. Existing populations survive in the northern Pacific and migrate from high-latitude temperate or polar waters after a summer of feeding, southward along North American and Asian coasts to breed in sheltered coastal waters. Although almost all gray whales migrate, whether reproducing or not, pregnant females move south earlier than males and then 80 or so days later return north again following behind the males and newly mated. Humpback and right whales follow similar patterns, but the behavior of rorquals, such as the blue (Balaenoptera mus-cidus), fin (B. physalus), and minke (B. acutorostrata and B. bonaerensis) whales, is more poorly known and, though seasonal, it is as yet unclear for many populations when and where breeding actually occurs.
The lives of odontocete cetaceans are less obviously compartmentalized and breeding takes place simultaneously with other activities. Detecting breeding seasons is consequently harder and is usually estimated from parameters such as the first appearance of neonates at sea, fetal maturity in stranded or captured animals, and seasonal changes in testes. From such studies, it appears that the majority of odontocetes extend their breeding activities over protracted seasons. Interestingly, those that remain in high-latitude areas tend to reproduce at the opposite time of year to neighboring mysticetes. Harbor porpoises (Phocoena phocoena) in the North Atlantic, for example, ovulate, mate, and give birth in spring and early summer, whereas seasonally sympatric humpback whales migrate south to breed in winter. Furthermore, within species, the specific timing of reproduction may vary by region or population. Common bottlenose dolphins (Tursiops truncatus), for example, show diffuse seasonal peaks in reproduction but these vary in their timing with location.
The reasons why mysticetes and odontocetes adopt such differing behavioral and physiological strategies toward the seasonality of reproduction remain poorly understood. Body size clearly allows the larger mysticetes and sperm whales to store sufficient reserves to forego feeding and dedicate time to breeding. Because most odontocetes are smaller, it is tempting to assume that they have less capacity to fast during a discrete breeding season; however, they are of similar body size or larger than the highly seasonally breeding pinnipeds. It therefore remains a possibility that odontocetes, and females in particular, have protracted breeding seasons, simply because other aspects of their fives allow it. For all cetaceans, it is likely that food availability, risk of predation, water temperature, and sea or river conditions are important in dictating which season is actually selected to breed.
B. Gathering Resources to Invest in Reproduction
At times outside of specific breeding seasons, mysticetes gravitate toward areas that maximize their potential for prey consumption. Migrations into productive, often high-latitude, areas are therefore common. The duration and rate of energy acquisition appear to be important in determining subsequent reproductive interval, ovulation rate, and fecundity. For odontocetes, particularly the smaller species, such migrations are less evident and suggest that their reproductive capabilities allow them to remain in their foraging areas year-round. A different strategy is found in the much larger sperm whales (Phtj-setermacrocephalns). Females remain in tropical or subtropical waters year-round, whereas the sexually dimorphic males migrate from productive high-latitude feeding areas toward the equator and their mates to breed. The extreme sexual dimorphism (with males weighing up to three times as much as females) may necessitate such migrations to regions especially rich in prey.
C. Locating a Suitable Place to Breed
Breeding in cetaceans can be broken down into three phases: giving birth, suckling young, and mating. Because gestation in most cetaceans is close to 12 months, these three activities generally occur at a similar time of year and are therefore often considered as if they were one event. However, the factors that influence each differ and thus the choice of breeding habitats may well represent a compromise for the individuals concerned. The processes of giving birth and suckling young may benefit from waters with low predator abundance while these characteristics will be of less importance for mating. Examples of differences in locations for these activities are rare, especially in odontocetes, where little is known about breeding site selection as a whole. One study of harbor porpoises in the North Sea found significantly higher proportions of calves in a specific coastal area relative to neighboring waters, although the reasons why this area was favored are as yet unknown. Mysticetes offer more concrete examples of breeding areas. Those most studied include humpback (Megaptera novaeangliae), gray, and right whales (Eubalaena spp.), which typically breed near coasts, with the latter species favoring sheltered shallow waters. As might be expected, they also offer some evidence of the differing requirements of raising young and mating, with females with newborn calves favoring slightly different areas to the other breeding individuals. It is unknown if breeding site availability is a significant factor limiting the size of behavior of cetacean populations.
Underlying all of the issues associated with mysticete migrations to breeding sites is the controversy over why the mysticetes migrate at all. Sheltered shallow waters are not unique to the tropics, and some mysticetes, such as the bowhead whale (.Balaena rmjsticetus), appear capable of breeding in the same polar waters in which they feed. Factors that pose direct benefits to adults do not appear to withstand scrutiny whereas the thermal constraints on calves do not seem likely when neonate mysticetes are larger than most adult odontocetes and are probably already tliermoneutral in colder waters. Instead, the possibility of calf predation by killer whales (Orcinus orca) might lie as the root cause of such enormous migrations for at least some of those species large enough to be capable of making them.
Gray whales appear to navigate to and from their breeding areas by following the coastal margins of their respective continents. In contrast, the north-south migrations of humpback whales seem to be deflected by coastlines, currents, and underwater topography rather dian guided by them. How these whales find locations such as the Hawaiian Islands each year in waters as large as the Pacific is still unknown. Use of celestial, acoustic, or magnetic markers are distinct possibilities.
D. Giving Birth
Few cetaceans births have been observed in the wild, but in captive odontocetes, most births are accomplished rapidly without direct assistance from conspecifics. However, there are several reports of animals seemingly helping in the birth process, either pulling the fetus or placenta clear of the birth canal. The frequency of such activities, if they occur at all in wild populations, is unknown. Because wild births have been observed so rarely, little is known about how females might reduce the risks of predation and separation in the moments after birth. The proficient swimming abilities of newborn calves permit mother and neonate to vacate an area rapidly where the birth occurred and so minimize the attentions of predators, while the social nature of cetaceans may permit increased predator detection and defense. Newborn calves adopt a swimming posture alongside their mothers permitting tactile communication, camouflage, and slipstreaming.
E. Investing in Young after Birth
Parental care in cetaceans is predominantly the responsibility of the mother, although male and female kin as well as unrelated females may provide additional care. The levels of non-matemal care vary considerably among species, being generally rare in mysticetes and more common in odontocetes. Pilot (Globicephala spp.) and killer whales offer some remarkable examples of care and nutritional investment that are notable not just within marine mammals but for all mammals.
1. Maintaining Contact and Providing Protection All cetaceans are born nutritionally dependent on their mothers, but with births occurring without the spatial certainties of land or ice, there is potential for calves to become separated from their mothers and therefore starve rapidly or become prey. For those species that suspend foraging during breeding, females can devote almost continuous attention to their calves. Species that continue to forage face a problem, as the diving abilities of calves may be insufficient to follow their foraging mothers. Babysitting among this latter group appears to be a common solution, with an apparent continuum among odontocetes. This can range from females of similar breeding status schooling with one another and presumably taking turns guarding calves or at least acting as a spatial point of reference, through to related females and their adolescent young accompanying mothers and their neonates in sperm whales. It may even extend to a system demonstrated by killer and pilot whales (apparently unique among mammals) of stable kin groups with neither male nor female dispersal but instead investment in raising their own (females only) and related offspring (females and males). Despite such behavioral safeguards, however, cetacean mobility makes separations between mother and calf inevitable. Individually specific calls are thought to be important in reuniting individuals in species such as the common bottlenose dolphin. However, such mechanisms take time to develop, and neonate mortality, although low compared with other mammals, is substantial.
Our understanding of cetacean reproductive behavior is undoubtedly hampered by the potential ambiguity of the behavior that can be observed. This is particularly prevalent in aspects involving the spatial proximity of individuals and apparently altruistic or cooperative behavior. The social complexities of cetacean societies and the considerable component that appears to be learned and practiced make context an essential component of any behavioral observation. Babysitting is an attractive and logical concept that has been described frequently, but the appearance of a calf with an adult animal other than its mother may result from other motives. Young inexperienced female Indian Ocean bottlenose dolphins (T. aduncus), for example, may temporarily kidnap calves and thereby improve their own maternal skills (Mann et al, 2000), whereas males and females of social mammals in general may benefit from capturing and killing another’s young.
2. Lactation and Weaning Cetacean calves do not suffer the constraints experienced by phocid seals, which need to rapidly transfer resources to the pup in order to resume feeding, nor do they endure the periods of maternal absence experienced by otariid pups. Instead female cetaceans take their mobile calves with them and are generally only separated for the length of a foraging dive. Thus calves have the opportunity to suckle frequently and match milk intake with energy expenditure and growth. Milk is transferred via two mammary teats, which are located in slits to either side of the genital opening about two-thirds of the way down the mother’s body on her ventral side. The presence of bristles on the rostra of neonate cetaceans is thought to help calves orient during suckling while a frilled margin to the tongue and muscular control of milk ejection likely aid efficient milk transfer.
Mysticete calves are generally weaned within a year of birth and, in migratory species, coincides with the pair reaching high-latitude feeding grounds. Weaning may precipitate separation of cow and calf but in its timing offers opportunities for the mother to train a calf in migration routes and the location of feeding areas, and potentially facilitate membership of feeding assemblages. Lactation in the majority of odontocetes is longer, and in some cases far longer, with weaning appearing to be gradual and occurring over a period of months or years. Lactose, for example, has been detected in the stomachs of sperm whales up to 13 years of age. Such long-term maternal investment suggests that manv components of odontocete development require a considerable period of learning and training. Foraging tactics in odontocetes are often performed in groups and, while it is unknown what proportion are cooperative or exploitative, their complexity is clear, as is the need for a high degree of in-terindividual coordination and practice. Calves may learn through observations or dedicated tutoring. The prolonged lactation and consequent investment in young allow calves to develop before facing nutritional independence. Bottlenose dolphins probably wean around 18 months after birth but remain closely associated with their mothers for at least 4 years. Young killer and pilot whales may never separate from their mothers, drawing comparison with elephant matriarchal societies where the eldest animals may function as long-term information stores and guardians for their offspring and offsprings offspring.
F. Locating and Selecting a Mate
1. Female Mating Behavior The number of offspring that female cetaceans produce in a lifetime varies. Groups such as the porpoises and baleen whales may give birth on an annual or biennial cycle and have the potential to produce between 12 and 20 offspring in a lifetime. Others produce much fewer. Some female killer whales, for example, may produce as few as 5 to 6 young in their entire lives. Whether 20 or 5, these numbers are small given the huge investment in time and resources that each calf receives, and therefore the choice of an appropriate mate to father them is a major component of the reproductive fitness for individual females.
In short, we have little solid information on how female cetaceans choose mates. In any species or population there are many potential junctures where a female may be making behavioral or physiological decisions, both before conception and afterward. In some instances, females may have the opportunity to simply select a particular male with which to copulate from a range of alternatives. Such a case has been proposed for humpback whales, where males may engage in communal display behaviors on the breeding grounds without showing any defense of resources. Females would have opportunities to approach males based on the quality of their displays. Female bottlenose dolphins are frequently seen being attended by single or alliances of males. While males may have opportunities to herd a female against her will, the females may also have the opportunity to reject or maintain that contact. Females have been observed rebutting the copulation attempts of males by fleeing or rolling upside down at the surface so that males do not have access to the females genital opening.
After copulation, females may have a range of behavioral and physiological options to influence the probability of conception. The number of subsequent males with which she mates would influence the probability of a particular male being the father. The large volumes of sperm produced by males of several cetacean species (see later) suggest that females mate with several males and that competition between the sperm themselves may be a frequent occurrence in such species. Mating repeatedly with a particular male would also bias the odds significantly. Whether an egg is available for fertilization is also critical and it appears that ovulation itself may be related to mating and therefore has the potential to be under the female’s control.
Even after fertilization has occurred there are opportunities to select whether to continue investment in a particular partner’s offspring. These may range from selection abortion, energetic investment in the fetus, and the subsequent level of parental care expended in the calf. At present we have little information to determine whether such behavioral and physiological decisions are made, but because evidence for such has been found in birds and terrestrial mammals, it is possible that such options are open to female cetaceans.
2. Male Mating Behavior The reproductive behavior displayed by males is a function of the social and physical environments in which they live and compete. As with the diversity of habitats and lifestyles exhibited by cetaceans, males of different species and populations show a huge range of tactics to maximize their reproductive potential. At a basic level, males should behave to optimize the number of their own sperm competing to fertilize a female’s egg and limit the number of those of competitors. Thus males may increase the probability of obtaining copulations by signaling their quality to females and competing males through physical or acoustic displays (e.g., postural displays in bottlenose dolphins, songs of humpback whales, sperm whale vocalizations), ornamentation or body scarring (teeth and scars in beaked whales), intermale combat (humpback whales), extreme body size (sperm whales), or simply tracking the long-distance migrations of females (humpback, gray, and right whales). Males may attempt to guard receptive or potentially receptive mates to reduce the probability of competitors mating and increase the number of copulations they can obtain themselves. The alliances formed between male bottlenose dolphins may be an example of such behavior where pairs or trios of males may trade off their exclusive access to a female in order to ensure that other males cannot gain mating opportunities. The potential absence of such alliances in some other populations of common bottlenose dolphins (Wilson et al, 1993) suggests that such tactics are context specific and influenced by factors such as the relative abundance of receptive females and potential for males to monopolize them.
Even once copulation has occurred, competition between males need not be over. Gray, bowhead, and right whales and harbor porpoises all have testes substantially larger than their body size would predict. Large quantities of sperm and the ability to copulate frequently may allow males to flush away or dilute the sperm and consequent reproductive chances of other males.
Males may also be able to increase the effective pool of receptive females by influencing the fate of other males’ calves. Infanticide is common among terrestrial mammals and is often carried out by males in order to force females to switch from investing resources into a previous calf not sired by themselves and to become reproductively receptive again. It is unknown if such behavior occurs in cetacean societies, but the violent deaths of young bottlenose dolphins in some populations suggest that males may exploit such options.
The long lives and intricate social organization of cetaceans, particularly odontocetes, also offer males the opportunity to increase their fitness, not by maximizing their potential to father offspring, but by investing in their kin. The lack of male dispersal in killer and pilot whales and the absence of interbreeding within pod members suggest that males may remain with their maternally derived relatives to provide care or protection and thus to increase their own inclusive fitness.
G. Mating
Cetaceans live in a three-dimensional environment that facilitates copulation from a variety of orientations. Common positions include ventrum to ventrum with the pair oriented in the same directions, or the male may mount the female from a nonparallel position. Intromission may last only a few seconds or far longer and involve vigorous thrusting or a more passive attitude. Mating may be preceded and followed by prolonged periods of courtship and petting. Mating episodes may be repeated over periods of minutes, hours, or days.
H. The Transition to the Nonbreeding Season
While copulation and parturition are generally seasonal in cetaceans, the investment in reproduction for females is an almost continuous process after reaching sexual maturity. Baleen whales that migrate from breeding grounds with neonate calves wean them on the feeding grounds. They may have several months of intensive feeding before returning to the breeding grounds to mate or be already pregnant following copulation the previous year. Female odontocetes frequently superimpose reproductive events by being both pregnant and lactating or suckling more than one generation of calf at the same time. Perhaps the most intriguing situation is demonstrated by female pilot whales, which appear to cease ovulating after age 40 and yet continue to lactate for well over another decade. In doing so, they have the potential to not only extend long-term care to their own offspring, but also influence the fate of their offspring’s own offspring.