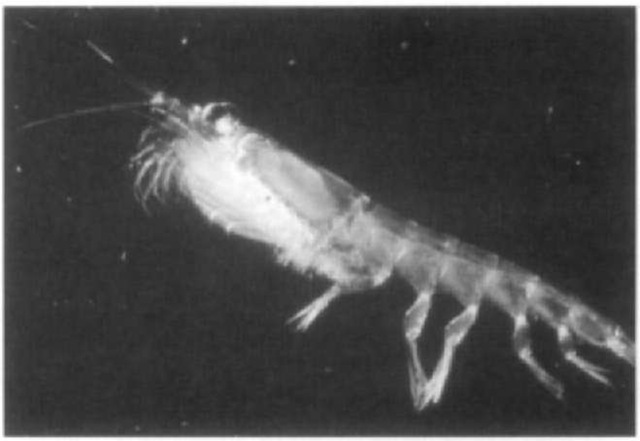
Euphausiids, or krill. have long been recognized as a critical element of the natural economy of the world’s oceans (Sars, 1885: Brinton, 1962: Marr. 1962; Mauchline and Fisher, 1969: Mauchline, 1980). Early fishery biologists repeatedly stressed the importance of various species of euphausiids as food for exploited fish and whale stocks (Lebour, 1924; Hick-ling, 1927; Hjort and Rund. 1929). Norwegian whalers referred to the euphausiids found in large numbers in the stomachs of whales caught in the North Atlantic as stor krill (or large krill. referring to Meganyctiphanes norvegica) and smaa krill (or small krill, referring to Thysanoessa inermis); the word “krill” is now used in reference to euphausiids in general (Mauchline and Fisher, 1969). Laws (1985) estimated that 190 million tons of Antarctic krill (Euphausia superba) were consumed annually by baleen whales in the Southern Ocean prior to their exploitation. It is estimated that the current populations of whales, birds, pinnipeds, fish, and squid consume 250 million tons of Antarctic krill annually (Miller and Hampton, 1989). Of the 85 species of krill, Mauchline and Fisher (1969) listed only nine of primary importance in terms of their distribution range, bio-mass, and dominance in the diets of vertebrate predators. They noted that these species constitute a large fraction of the plankton where they are found and that their biomasses are largest at high latitudes. In addition to their numbers, the habit of euphausiids to form large swarms makes them particularly important as prey to marine vertebrates (Fig. 1).
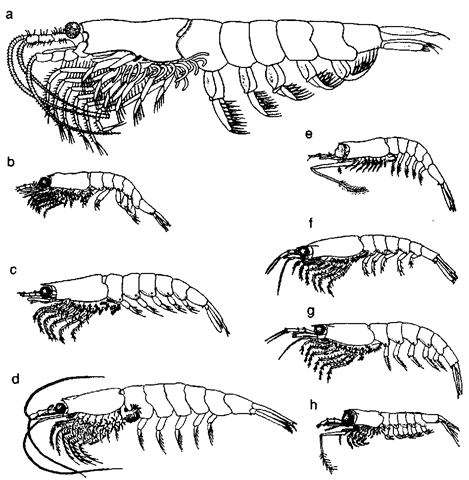
The krill species considered most important to the tropho-dynamics of marine ecosystems are M. norvegica, T. rasehii, and T. inermis in the North Atlantic Ocean; E. pacifica, T. in-ermis, T. rasehii, T. longipes, and T. inspinata in the North Pacific Ocean; and E. superba, E. crystallorophias, and T. macrura in the Southern Ocean. Mauchline and Fisher (1969) list another seven species of importance in more restricted geographical areas and/or seasons: Nyctiphanes couchii in the North Atlantic Ocean; T. spinifera and E. similis in the North Pacific Ocean; JV. capensis near the southern part of Africa: JV. australis and Pscudoeuphausia latifrons from Western Australia to New Zealand; and E. vallentini in the Southern Ocean. In addition, the following seven species are often cited in predator diet samples from restricted locales and time periods: Ne-matocelis megalops in the North Atlantic; E. recurva, E. lucens, E. hemigibba, T. gregaria, E. spinifera, and JV. megalops from Western Australia to New Zealand: E. recurva, E. lucens. and T. gregaria near the southern part of Africa: and JV. simplex along the western coast of North America (Fig. 2).
Figure 1 Euphausia superba.
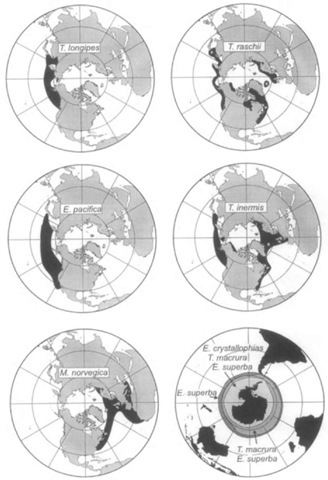
While many of these species are broadly dispersed, they exhibit their highest densities in areas of enhanced seasonal primary and secondary production. These areas include eastern boundary currents, coastal and oceanic upwelling regions, and sea ice edge zones, as well as estuaries, fjords, and small-scale eddies where physical mechanisms may enhance the aggregation of krill. It is not surprising therefore to find krill predators, including baleen whales and crabeater seals, concentrated in these areas as well (Fig. 3).
Krill species differ in their geographic distribution, body size (ranging from less than 1 cm to 14 cm), and longevity (ranging from less than 1 year to as many as 10 years) but share many other characteristics that contribute to their importance as prey for baleen whales. Furthermore, baleen whales have not shown strong species or size selectivity among krill when foraging in an area where more than one species and/or developmental stage is present. Krill are therefore described here in general terms with species-specific reference only where appropriate.
I. General Morphology and Life History
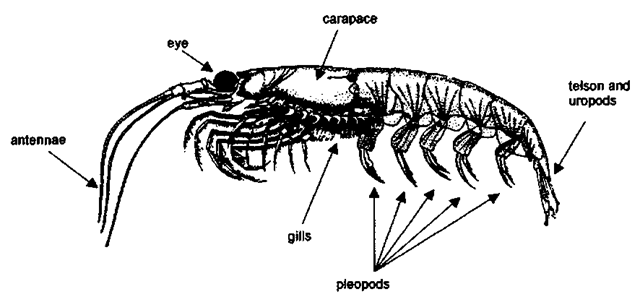
The body plan of krill (Fig. 4) is divided into two main regions: the ceplialotliorax and the abdomen. The ceplialotliorax, a fused head and thorax, contains the internal organs, including the digestive system, the heart, and gonads. It is about one-third of the body length and is covered by a thin shell or carapace. The muscled abdomen is made up of six segments ending with a telson and two pair of uropods, which together form a fan shape at the tail. At the head there are a pair of eyes and two pair of antennae with tactile and olfactory sensors; excretory organs open near the second set of antennas. The mouth is made up of several parts whose function is to filter, macerate, and manipulate food prior to ingestion. Six to eight pairs of limbs are connected to the thorax and are used to filter particles out of the water and pass them to the mouth. Unlike decapod crustaceans (crabs, lobsters, prawns, shrimps) the gills of krill are exposed, hanging below the carapace. The first five abdominal segments each have a single pair of limbs (pleopods) attached, which are used for swimming; the sixth abdominal segment has no appendages. On a mature adult male the first pair of pleopods is modified to form a petasma, which is used during copulation to clasp and transfer spermatophores to the female. The thelycum, or female copulatory organ, is located on the anterior underside of the thorax near the opening of the oviducts.
Figure 2 Scale drawing of the eight most important krill species: Euphausia superba (a), E. Pacifica (b), E. crystallorphias (c), Meganyctiphanes norvegica (d), Thysanoessa niacrura (e), T. inenriis (f), T. raschii (g), and T. longipes (h).
The exoskeletons of krill are translucent, allowing a view of the internal organs, including the heart, stomach, and he-patopancreas, which is often colored dark green or red. Krill are also luminescent with light-emitting photophores located at the bases of their pleopods, near the thelycum, close to the mouth and in the eye stalks. The photophores are a deep red color but emit electric blue light in the water. Many species are also pigmented with red chromatophores that expand when the animal is stimulated. As a result, swarms of krill often appear to be bright red, particularly when under attack by a predator. The guano of krill-eating birds is often pink in color, and the feces of krill-eating marine mammals are characteristically dark red.
As krill mature sexually, males elaborate packets of sperm called spermatophores and females develop clusters of eggs or broods. During spawning the male grasps the female with his petasmae and transfers spermatophores to her body where they adhere in the vicinity of her thelycum. Among the various species of krill, brood size ranges from tens of eggs to several thousand, and some species have been observed to spawn several broods during a single breeding season. When a female releases a brood of eggs, they are fertilized by spermatozoa now liberated from the spermatophores. For some species the female carries the fertilized eggs in brood pouches until they hatch, thereby protecting them from predation. For most species, however, eggs are released into the open sea. In some cases the eggs are neutrally buoyant, but often they are heavier than water and sink before hatching into nauplius larvae, which in turn develop and molt through a series of larval stages, each resembling the adult morphology more than the previous stage. In the case of E. superba, a brood of 10,000 fertilized eggs may be released by a single female in a near-surface swarm of spawning adults; the eggs sink to depths of greater than 1000 m, incubate, and hatch. The nauplius has no swimming appendages and continues to sink as it grows, molts, and gives rise to more advanced larval forms. Once it can swim the larva begins its ascent into the surface waters, progressing through several more molts and ultimately emerging as a calyptosis larva. Calyptoses continue to eat, grow, and molt through additional stages in preparation for the winter when food is less available. Sometime in the late winter or early spring the larvae finally metamorphose into juvenile krill, but it may be as long as another year before they are ready to spawn themselves. In the case of E. pacifica, this process is compressed to a few months with spawning occurring during the spring and recruitment into the adult population occurring during the fall
Figure 3 Northern and Southern Hemisphere maps showing dispersion of important krill species. Redrawn from Mauchline and Fischer (1969).
Figure 4 General krill body plan.
Except for rich fat stores invested in developing eggs, larval and postlarval krill do not elaborate high levels of fat reserves. Consequently, they must eat constantly in order to offset the energy costs of swimming, growth, and reproduction. In addition, krill periodically shed their exoskeletons throughout their life, adding substantially to their energy requirements. Krill are generally thought to be filter-feeding herbivores, grazing on phytoplankton in the surface layers of the ocean. Many species, however, are reported to be omnivorous, filtering and/or capturing copepods and other small zooplankton. E. superba has been observed in the cavities and cracks on the underside of winter sea ice, presumably feeding on interstitial ice algae. Krill growth and reproductive activity have been directly linked to available food supplies. Negative growth and regression of sexual characteristics have been observed in several species and related to lowered availability of food.
II. Swarming
Krill are heavier than water and must swim continually in order to maintain their position. They aggregate into dense swarms, which can take on a variety of shapes from discreet balls to extensive layers. The swarms may range from 1 m thick to several tens of meters and may extend horizontally tens of meters to several thousand meters. Individual animals appear to be in constant movement, and a sharp gradient in density is often observed at the periphery of a swarm. Within the swarm, volumetric densities may range up to several thousand animals per cubic meter. Near the shelf break surrounding islands in the southwest Atlantic sector of the Southern Ocean it is not uncommon to observe large swarms of E. stiperba, each estimated to contain several thousand tons of krill. Most krill species migrate vertically each day, moving into the upper waters at night and dispersing and moving downward just before dawn and aggregating into denser concentrations. It is generally thought diat this behavior is the result of a trade-off between avoiding predation (dense swarms deep in the water during the day) and maximizing feeding efficiency (dispersed individuals in the more particle-rich surface water at night). Although this is a regular pattern, vertical migration behavior varies between species and within a species depending on location and season. Daytime surface swarms have been observed for several species and often contain reproductively mature individuals.
The highest densities of krill have been reported near areas of strong vertical mixing and enhanced primary production. These include coastal upwellmg zones, ocean frontal boundaries, and topographic features that interrupt or modify currents such as continental shelf breaks, underwater canyons and escarpments, and seamounts. Krill swarms also tend to aggregate in areas of water flow discontinuity such as eddies and sheer zones between opposing currents.
III. Recruitment Variability
Recruitment of young animals into adult euphausiid population is highly variable in space and time. The production of spawn and the survival of larvae may vary widelv within the distribution range of a species as well as between reproductive events. In his review of euphausiid life histories, Siegel (2000) noted that most species reduce their growth phase and extend their reproductive phase toward the center of their distribution ranges. Closer to their distribution limits, krill put more time into growth and less into reproduction.
There is no apparent relationship between stock size and the production of new recruits for most species studied. Relatively large adult stocks can produce few new recruits, and small adult stocks are capable of producing enough new recruits to increase the stock abundance severalfold. The intensity of spawning, sunival of eggs and larvae, and the rate of growth have been shown to vary widely between years for several species, resulting in large year-to-year variability in abundances. lnterannual variability in the abundances of E. pacifica and N. simplex off the west coast of North America has been estimated as 10-fold, 25-fold for T. inermis in the Barents Sea, and 5- to 60-fold for M. norvegica at different parts of its range in the North Atlantic.
Recruitment success is affected by exogenous factors, which act to enhance adult reproduction, survival of eggs, and growth of larvae. The best documented of these is the influence of coastal upwelling, which enhances primary production and the subsequent growth and maturation of young krill. Temperature affects the incubation rate of eggs and the growth rate of larvae, exposing them to longer or shorter periods of predation. Fluctuations in currents may also transport animals into unfavorable areas. Near the Antarctic Peninsula, E. superba spawn earlier in the spring and for a longer period following winters of extensive sea ice development; their larvae enjoy a higher survival rate if sea ice is extensive during the following winter (Loeb et al, 1997). Four to 5-year cycles are apparent in the seasonal extent of sea ice and the recruitment of krill in this region of the Southern Ocean. The postulated affect of seasonal sea ice is to provide a refuge, access to a wintertime food source (ice algae), and to inhibit rapid springtime population growth of a potential competitor to krill, Salpa thompsoni (Loeb et al, 1997). Salpa thompsoni is a pelagic tunicate and obligate filter feeder, which requires open water access to springtime phyto-plankton blooms in order to reproduce.
IV. Foraging Tactics of Baleen Whales and Crabeater Seals
The two characteristics of euphausiids described earlier— an immediate response, in terms of individual growth and reproductive output, to favorable conditions and highest densities in predictable locales—allow efficient exploitation of krill by baleen whales.
In general, baleen whales migrate between high-latitude summer feeding grounds and low-latitude winter breeding and calving grounds. Exceptions are bowhead whales (Balaena mtjs-ticetus), which are restricted to Arctic regions, and Bryde’s whales (B. edeni). which usually range from subtropical to temperate waters. Blue, fin, and sei whales (Balaenoptera musculus, B. physalus, and B. borealis) tend to migrate in offshore waters while gray (Eschrichtius robustus), right (Eubalaena spp.), and humpback (Megaptera novaeangliae) whales tend to use a more coastal migration route. Adult whales are thought to feed less during migration than immature or undernourished animals. Off the western coast of North America, blue, fin, Biyde’s, and humpback whales have been observed feeding on euphausiids aggregated along underwater escarpments and canyons during both winter and summer. The location and timing of whale foraging follow the appearance of high densities of euphausiids and tend to progress from south in the winter to north in the summer. In recent years, aggregations of euphausiids and foraging whales have been a predictable event in the Gulf of California during late winter, near underwater seamounts and canyons off northern California in the summer, and along the shelf break surrounding the Channel Islands in the fall.
Actively feeding whales have been observed to lunge through surface swarms of krill, engulfing large quantities of water and distending their bellies, before expelling the water and extruding as much as several hundred kilograms of krill (Fig. 5). Similar feeding behavior on subsurface swarms has been inferred from acoustic records of krill layers superimposed with dive tracks simultaneously recorded by instruments attached to foraging whales. There are many reports of humpback and fin whales herding and concentrating their prey before lunging through an aggregation of krill. Bryde’s and minke whales (B. acutorostrata and B. bonaerensis) have also been observed gulping large quantities of aggregated euphausiids. Foraging by right and bowhead whales has been described as skimming a continuous stream of water rather than gulping; this behavior may be more efficient with dispersed prey (Nemoto. 1970). Sei and gray whales appear to use both methods.
Figure 5 Blue whale lunging through a subsurface krill layer, from an animation.
Despite their name, crabeater seals (Lobodon carcinophaga) eat very little other than krill. They are found in the sea ice zone in the Southern Ocean and constitute 50% by number (75% by weight) of the world pinniped population. Crabeaters have lobed cusp teeth with spaces between them. It is presumed from the shape of the mouth, tongue, an spacing between the teeth that crabeater seals engulf a portion of an aggregation of krill and then strain the water similar to a baleen whale. Crabeaters tend to feed at night when krill are in the upper layers and more dispersed than during the day.
V. Marine Mammal Diets and Euphausiid Consumption by Ocean Basin
A. North Pacific
Blue whales in the eastern North Pacific, foraging from British Columbia to the Californias, feed principally on three species of krill in the California Current; E. pacifica and Thysanoessa spinifera, the more inshore species, which is replaced by Nyctiphanes simplex moving south (Fig. 6). Fin whales have been observed feeding from the Gulf of California to the northern parts of the Bering Sea from April to September, respectively. During the late winter and spring, fin whales feed on N. simplex in the southern portion of their foraging range: moving north in the summer they feed on E. pacifica, T. rasehii, T. longipes, and T. inermis. Fin whales have also been observed to feed on copepods, with the change in prey type related to changes in local relative densities of prey. During the summer months, sei whales consume a variety of euphausiid species, including T. gregaria, E. pacifica, E. recurva, E. diomedeae, E. tenera, T. inermis, T. spinifera, N. dijficilis, and N. gracilis.
Figure 6 A blue whale swimming through a stirface swarm of krill.
South of Japan less than 2% of the sei whale diet has been reported to consist of fish, whereas prey species consumed near the Aleutian Islands include copepods, amphipods, decapod crustaceans, fishes, and squid. In comparison to blue whales and fin whales, sei whales appear to be more opportunistic feeders, willing to switch prey type more readily in response to local availability. Bryde’s whales have been observed consuming E. similis, N. difficilis, and T. gregaria as well as amphipods, cope-pods, and fish in the western Pacific and both euphausiids and fish in the Gulf of California. Humpback whales have been observed foraging on euphausiids, including E. pacifica, T. raschii, T. longipes, and T. spinifera, from Southeast Alaska to Baja California, although a substantial part of their diet includes clupi-oid fish as well. Bowhead whales forage primarily on T. raschii and T. inermis in the Bering and Beaufort Seas during summer and fall, although copepods, mysids, and amphipods also form a part of their diet. Minke whales have been observed foraging on euphausiids, but appear to prefer fish throughout the North and northeastern Pacific. Gray whales are thought to consume primarily benthic amphipods in the Bering Sea during the summer months, although there are reports of gray whales consuming T. raschii in the Bering Sea and E. pacifica off northern California. Prey selectivity among ringed seals (Pusa hispida) appears to be dependent on seasonality and location. Ringed seals have been reported to eat T. raschii, T. longipes, and T. inermis in offshore waters in the Northern Hemisphere in spring and summer when krill abundance is greatest; in the winter they consume Arctic cod and other fish species in inshore waters.
B. South Pacific
Bryde’s whales have been observed feeding on E. diomedeae, E. recurva, and T. gregaria and occasionally fish in the Coral Sea (western South Pacific) during the austral spring. In the eastern South Pacific, Bryde’s whales consume euphausiids during the austral summer between 35° and 40° south latitude. Humpback whales have been observed off the east and west coasts of Australia feeding on euphausiids, including E. hem-migibba, P. latifrons, and E. spinifera.
C. North Atlantic
Fin whales feed primarily on M. norvegica, T. inermis, and T. raschii during the summer months, switching between prey species in response to local availability. Minke whales consume T. inermis and M. norvegica in the North Atlantic where euphausiids form a much larger portion of the diet than in the Pacific. North Atlantic right whales (Eubalaena glacialis) feed primarily on copepods, although consumption of euphausiids has been observed particularly when associated with copepods. Harp seals (Pagophilus groenlandicus) feed on a variety of prey, including decapods, amphipods, euphausiids, and pelagic fishes; however, newly weaned pups and young seals have been reported to feed mainly on Thysanoessa species.
D. Indian Ocean
Fin and minke whales have been observed feeding on euphausiids in the southwest Indian Ocean during their spring and fall migrations to and from the Southern Ocean; prey species include E. recurva, E. lucens, T. gregaria, E. spinifera, N. capensis, and E. diomedeae. Bryde’s whales forage on these euphausiids species as well in the southwest Indian Ocean. Near Durban, South Africa humpback whales have been observed feeding on E. recurva and T. gregaria, and a single pygmy blue whale (B. musculus brevicauda) was reported feeding on E. recurva and E. diomedeae. Sei whales were observed to consume euphausiids, as well as copepods, amphipods, pteropods, and fish.
E. Southern Ocean
Fin and minke whales consume several species of krill in the Southern Ocean throughout the austral summer. Species preference appears to be related to local availability with T. macrura and E. vallentini more prevalent in the diets of animals foraging in open waters and E. frigida and E. cnjstal-lorophias more prevalent near the continental shelf and ice edge regions. The numerically dominant euphausiid in the Southern Ocean, E. superba, is consumed in all areas. Southern right whales (E. australis) have been observed foraging on E. stiperba in the Atlantic sector of the Southern Ocean. Humpback whales have been frequently observed foraging on E. superba in bays and fjords along the Antarctic Peninsula. Crabeater seals consume E. superba and E. crystallorophias in the sea ice zone and in coastal fjords and bays; Antarctic sil-verfish have been reported as seasonal constituents of their diet but krill has been estimated to provide over 90% of their prey requirements. Much smaller portions of the diets of leopard (Hydrurga leptonyx), Ross (Ommatophoca rossii), Antarctic (Arctocephalus gazella), and subantarctic (A. tropicalis) fur seals have been reported to be composed of krill.
From these observations, some generalizations may be drawn: (1) blue and fin whales appear to have a higher preference for euphausiids than minke, humpback, or bowhead whales; (2) sei and Biyde’s whales appear to be more opportunistic feeders; (3) gray whales and northern right whales prefer prey other than euphausiids but will consume them; (4) crabeater seals have a higher preference for euphausiids than other seals in the Southern Ocean; and (5) ringed and harp seals in the Northern Hemisphere include euphausiids in their diets during certain times of the year and life cycle.
Gross estimates of the consumption of euphausiids by marine mammals are summarized in Tables I and II. Estimates of stock abundances were obtained from working papers and reports of the International Whaling Commission, reports from the U.S. National Marine Fisheries Service, and the primary literature. In some cases, no reliable estimates are available and broad ranges were used. Daily ingestion rates for baleen whales during the feeding season were estimated from energetic requirements as a function of body weight following Sigurjonsson and Vikings-son (1997). A daily ingestion rate for seals was estimated as 7% of body weight. Average body weights, the percentages of euphausiids in the diets, and the caloric value of euphausiids (0.93 kcal/g) were taken from the primary literature. The duration of the feeding season was assumed to be 180 days for Northern Hemisphere baleen whales and seals, 120 days for Southern Hemisphere baleen whales and Antarctic and sub-Antarctic fur seals, and 335 days for crabeater, leopard, and Ross seals.
TABLE I
Consumption of Euphausids by Whales
|
Ocean basin |
Whale species |
Abundance |
Average body weight (tons) |
Summertime ingestion rate (Uf kcal/day) |
Feeding period (days) |
% krill in diet |
Krill consumed (10? tons) |
|
North Pacific |
Blue whale |
3,000-4,000 |
69.2 |
2136 |
180 |
100 |
1,240-1.654 |
|
Fin whale |
14,600-18,600 |
42.3 |
1452 |
180 |
80 |
3,282-4,182 |
|
|
Sei whale |
13.000-13,000 |
19.9 |
805 |
180 |
80 |
1,621-1,621 |
|
|
Bryde’s whale |
34,500-45,500 |
13.2 |
584 |
180 |
40 |
1,559-2.056 |
|
|
Minke whale |
30,000-32,000 |
5.3 |
284 |
180 |
70 |
1,153-1,229 |
|
|
Humpback whale |
5,000-6,000 |
31.8 |
1161 |
180 |
60 |
674-809 |
|
|
Bowhead whale |
8,000-10,000 |
80.0 |
2392 |
180 |
80 |
2,963-3,704 |
|
|
Gray whale |
25.000-27,000 |
25.0 |
962 |
180 |
5 |
233-251 |
|
|
Northern right whale |
400-600 |
55.0 |
1784 |
180 |
25 |
35-52 |
|
|
|
|
|
Total |
12,760-15,559 |
|||
|
North Atlantic |
Blue whale |
750-1,300 |
69.2 |
2136 |
180 |
100 |
310-538 |
|
Fin whale |
45,000-50,000 |
42.3 |
1452 |
180 |
80 |
10,117-11,241 |
|
|
Sei whale |
9,000-13,000 |
19.9 |
805 |
180 |
80 |
1,122-1,621 |
|
|
Minke whale |
120,000-182,000 |
5.3 |
284 |
180 |
70 |
4,610-6,992 |
|
|
Humpback whale |
10,000-11.000 |
31.8 |
1161 |
180 |
60 |
1,349-1,483 |
|
|
Northern right whale |
300-350 |
50.0 |
1656 |
180 |
25 |
24-28 |
|
|
|
|
|
Total |
17.532-21,903 |
|||
|
South Hemisphere |
Blue whale |
600-800 |
83.0 |
3708 |
120 |
100 |
287-383 |
|
Pygmy blue whale |
2,000-6,000 |
68.9 |
3205 |
120 |
100 |
827-2,481 |
|
|
Fin whale |
10,000-20,000 |
48.0 |
2415 |
120 |
100 |
3,116-6,232 |
|
|
Sei whale |
35.000-45.000 |
17.5 |
1096 |
120 |
80 |
3,960-5,091 |
|
|
Bryde’s whale |
78,000-108,000 |
13.2 |
879 |
120 |
40 |
3,538-4.899 |
|
|
Minke whale (two species) |
650,000-950,000 |
7.0 |
535 |
120 |
100 |
44.858-65,561 |
|
|
Humpback whale |
15.000-16,000 |
26.5 |
1517 |
120 |
100 |
2,936-3,131 |
|
|
Southern right whale |
6,500-7,500 |
55.0 |
2687 |
120 |
100 |
2,253-2,600 |
|
|
|
|
|
Total |
61,775-90,379 |
Although Tables I and II are based on several simplifying assumptions, some general conclusions may be drawn. Total consumption of euphausiids by marine mammals is on the order of 10-20 million tons per year in the North Pacific, 15-25 million tons per year in the North Atlantic, and 125-250 million tons per year in the Southern Hemisphere, with the bulk of the latter portion consumed in the Southern Ocean. In the North Atlantic, the largest portion is consumed by fin whales followed by minke whales. In the North Pacific, consumption is distributed more evenly, with fin and bowhead whales consuming the most, followed by blue, sei, Bryde’s, and minke whales, all of which consume similar portions. In the Southern Hemisphere, comparable proportions of euphausiids are consumed by crabeater seals and baleen whales. Of the estimated total krill consumption by baleen whales in the Southern Ocean, minke whales (two species) consume approximately two-thirds. Crabeater seals consume more krill than any other marine mammal population in the world.
These crude calculations suggest that baleen whales consume a substantial amount of euphausiids; moreover, their food requirements must have been several times more prior to commercial whaling. Unfortunately, there is little information on which to judge whether krill production was higher prior to the onset of commercial whaling or whether other krill predators (e.g., crabeater seals) benefited as a result of the decline in baleen whale stocks.
What is more apparent is that krill abundance can vary dramatically over relatively short periods of time and that baleen whales have adapted to this variability. Their size and ability to accumulate substantial energy stores allow them to integrate over large distances and periods of time in their search for food.
TABLE II
Consumption of Euphausiids by Seals
|
Ocean basin |
Seal species |
Abundance |
Average body weight (tons) |
Summertime ingestion rate (10? kcal/day) |
Feeding period (days) |
% krill in diet |
Krill consumed (103 tons) |
|
Northern Hemisphere |
Ringed seal |
6,000,000-7,000,000 |
75 |
5.3 |
180 |
25 |
1418-1654 |
|
|
Harp seal |
100,000-400,000 |
130 |
9.1 |
180 |
25 |
41-176 |
|
|
|
|
|
|
|
Total |
1458-1830 |
|
Southern Hemisphere |
Crabeater seal |
15,000.000-30.000,000 |
220 |
15.4 |
335 |
94 |
72,742-145.484 |
|
Leopard seal |
300,000-500,000 |
275 |
19.3 |
335 |
37 |
716-1,193 |
|
|
|
Antarctic fur seal |
1,000,000-1,500,000 |
50 |
3.5 |
120 |
50 |
210-315 |
|
|
Sub-antarctic iur seal |
300.000-500,000 |
85 |
6.0 |
120 |
50 |
107-179 |
|
|
Ross seal |
125.000-225,000 |
175 |
12.3 |
335 |
10 |
51-92 |
|
|
|
|
|
|
|
Total |
73.826-147,263 |
Their longevity allows them to spread reproductive effort over several years. It is reasonable to expect, however, that the supply of euphausiids will not be sufficient in all years to meet total energy requirements and that reproductive success and population growth among krill-dependent baleen whales may vary from year to year in response to the availability of their prey.
VI. Anthropogenic Effects
The production of euphausiids can be very sensitive to environmental conditions. This raises two concerns with regard to the influence of human activities. The first is that highly productive euphausiid populations may be able to sustain large fisheries. The second is that climatic change (whether man-induced or not) mav affect the frequency of environmental conditions that are favorable for reproductive success. Because these are relatively recent developments, we cite three studies as entries into a larger body of literature.
Fisheries on euphausiids have the potential of being the largest in the world. In their review of krill fisheries, Nicol and Endo (1999) described the harvest of E. pacifica off the coasts of Japan and western Canada, T. inennis off the coasts of Japan and eastern Canada, E. nana off the coast of Japan, T. raschii and M. norvegica off the coast of eastern Canada, and E. su-perba in the Southern Ocean. In recent years, the harvest of E. pacifica off Japan (ca. 60,000 tons per year) and E. supcrba in the Scotia Sea region of the Southern Ocean (ca. S0.000 tons per year) comprised over 90% of the world harvest of euphausiids. Nicol and Endo (1999) noted that these yields are well within their theoretical potentials, although expansion of the coastal fisheries is unlikelv because of ecological, economic, and political considerations. They further note, however, that as conventional fisheries decline and demand for krill as aqua-culture feed increases, fishing pressure is likely to shift to E. superba in the Southern Ocean where current harvests are far below current estimates of sustainable yields.
Evidence suggests that the production of euphausiids may be affected by long-term climatic change. Warming of the surface waters of the California Current since the mid-1970s has been accompanied by a reduction in the depth of the thennocline, reduced nutrient input via coastal upwelling, reduced primary production, and an overall decrease in macrozooplankton biomass by as much as 80% (Roemmich and McGowan, 1995). Euphausiids are the dominant taxa in the macrozooplankton fauna of the California Current and have shown decreased abundances during warm (El Nino) years and increased abundances during cold (La Nina) years. A 50-year warming trend in the Antarctic Peninsula region has been associated with a decrease in the annual production of sea ice. Loeb ct al. (1997) correlated the reproductive success of E. supciiia with the wintertime extent of sea ice and suggested that the warming trend may cause a decrease in the frequency of strong year classes of Antarctic krill, a decrease in the mean population abundance of krill, and a change in the carrying capacity of vertebrate krill predators in the region.