Genetics constitute the study of heredity and variation of % inherited characteristics. In the case of genetic analyses of natural animal populations at the level of organisms or above (e.g., populations or phyla), most studies draw their inferences from relative differences in consanguinity (i.e., kinship or relatedness). However, in the case of natural populations, we usually possess little or no prior knowledge as to the exact degree of relatedness among the individuals that we are comparing (whether from the same or different species). Hence, the primary task becomes to obtain an accurate estimate of the degree of relatedness among individuals sufficient for the purposes of the specific study.
In principle, the relative degree of relatedness among organisms is estimated from, and positively correlated with, the proportion of shared inherited characters. It is possible to use any trait of an organism toward this end; however, the further removed from the locus encoding the trait (i.e., the DNA itself), the higher the chance that external factors may have altered the phenotypic expression of a trait. Hence, while relatedness can be estimated from morphological characters and a single morphological character might represent many loci, the phenotypic expression might be influenced by extrinsic factors, such as environmental or physiological variables, to an unknown extent. In contrast, the composition of most cellular components is not susceptible to such extrinsic variables and thus the interpretation of the observed variation can be directly linked to the state of the encoding locus, the genotype. This explains why biochemical/molecular methods were so readily adopted and applied to estimate genetic and phylogenetic relationships when these first emerged in the mid-1960s. Until the 1980s the biochemical/molecular methods applied to natural populations were mainly indirect, e.g., the most widely employed method, isozyme electrophoresis, detects differences in the overall electric charge of enzymes due to amino acid substitutions. An important limitation of isozyme analysis is that the proportion of the genome, which encodes for detectable proteins, is veiy small, and only a subset of amino acid substitutions will yield a change in the overall electric charge of the enzyme. In addition, homoiotherm organisms (birds and mammals) have a reduced level of isozyme variation compared to poikilotherm animals and plants.
Despite these limitations, a large number of studies have been conducted based on isozyme electrophoresis, providing novel and valuable insights. Interested readers should consult the works of Wada and Danielsdottir, both of whom have undertaken extensive isozyme-based studies of various species of cetaceans.
The most basic source of genetic data, the nucleotide sequence of the genome itself, became accessible in a practical manner due to a series of technical advances during the 1980s culminating with the development of the polymerase chain reaction (PCR) by Mullis and co-workers in 1987. The PCR technique permits simple in vitro amplification of any specific nucleotide sequence if the nucleotide sequence of the flanking regions is known. Once amplified, the exact nucleotide sequence of the locus is readily determined. Today, PCR-based analyses of DNA sequences are the predominant methods used in genetic studies of marine mammals, which is why this article relies on examples based on the analysis of DNA sequences rather than isozymes or morphological characters.
I. Obtaining Tissue Samples
A prerequisite for DNA-based methods is, naturally, DNA. The most common source is samples of soft tissue from which the DNA subsequently is extracted. Soft tissue samples are readily available from dead animals, e.g., stranded or killed specimens. However, it is often scientifically or ethically desirable to obtain samples from free-ranging, live animals. The advantage of PCR-based techniques is that only a minute amount of target DNA is required. Adequate amounts of DNA are contained in skin biopsies, sloughed skin, hair, and even feces, which can be collected from free-ranging marine mammals with relative ease.
The sensitivity of PCR-based methods also enables the use of historical samples, such as hair from old furs, baleen, or even dried blood obtained from old log books. However, the quality of DNA extracted from such historical samples is usually inferior and obtained in much lower concentrations than DNA extracted from current samples. The same is usually true for DNA extracted from fecal or similar degraded samples. The low concentration and often highlv degraded DNA obtained from such samples necessitate additional precautionaiy measures to prevent contamination as well as repeated analyses to ensure that a correct genotvpe is obtained.
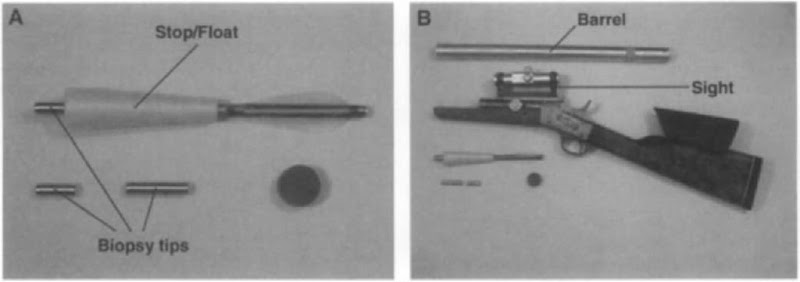
Tissue samples from free-ranging animals can be collected by invasive and noninvasive techniques, each with their respective advantages and disadvantages. Invasive techniques, such as the collection of skin biopsies, enable a directed sampling scheme. This implies that, conditions permitting, skin biopsies can be collected from individuals relevant to the specific objective and a biopsy can be linked to a specific individual. Multiple biopsy systems have been developed to collect skin biopsies from marine mammals, all principally consisting of a delivery unit, such as a crossbow or gun, and a projectile unit, usually an arrow. The projectile unit carries the biopsy tip and a stop to limit the depth of penetration, which may act as a float as well. The biopsy tip is typically a simple hollow tube of stainless steel with one or more barbs retaining the sample. Systems of various kinds and ranges have been developed, the most recent is the long-range system developed by Dr. Finn Larsen at the Danish Institute for Fisheries Research with which a skin biopsy was collected from a blue whale, Balaenoptera musculus, at a distance of approximately 70 m (~210 feet, see Fig. 1). Skin biopsies from pinnipeds or smaller odontocetes are usually collected when the animals haul out on land or bow ride using a pole onto which a biopsy tip is mounted. Invasive sampling techniques are at times viewed as intrusive and thus undesirable. In order to investigate such concerns, data have been collected during biopsy sampling in order to detect possible adverse effects. To date the only discernible effects appear to be short term and may be equally attributable to the close approach of the boat necessary to collect a sample.
The alternative, noninvasive sampling methods are usually of a more opportunistic and random nature, which may prohibit the pursuit of some research objectives. For cetaceans, the most common kind of noninvasive sample is sloughed skin. The outer epidermis in cetaceans differs from most other mammals by the lack of dead keratinized cells and consists mostly of live cells complete with nuclei and mitochondria: the organelles that host the two cellular genomes. There is considerable variation among cetacean species in terms of the amount and how often they slough skin. Sperm whales have been observed to slough massive amounts of skin, whereas other species, such as fin and minke whales, rarely slough any skin. The main disadvantage when collecting samples such as sloughed skin is the opportunistic nature of the samples and the difficulty in linking a specific sample to a particular individual during multi-individual sightings, which may influence the pursuit of some objectives. In addition, the quality and quantity of DNA extracted from such samples are more variable than those obtained from skin biopsies.
Figure 1 The “Larsen” long-range skin biopsy system. (A) The projectile unit with biopsy tips and concave stop, which acts as afloat as well. (B) The delivery system (a Remington rolling block system rifle), complete with barrel and sighting aid.
Genomic DNA has also been extracted successfully from fecal plumes collected in the water column from dugong and dolphins, which contain epithelial cells from the intestinal tract. Among pinnipeds, the most common type of noninvasive samples is fecal samples, typically collected from haul-out sites on land. In bears, sloughed hair has proven an excellent source of noninvasive samples where the DNA is extracted from the root cells. In the case of bears, a simple, yet highly effective sampling scheme has been utilized based on “hair traps” with scent lures to attract bears and barbed wire that passively collects hair samples.
Samples are usually preserved by freezing with or without conservation buffer. Commonly used conservation solutions are 70-96% ethanol or distilled water saturated with sodium chloride and 20% dimethyl sulfoxide (DMSO).
II. Commonly Analyzed Loci
As mentioned earlier, genetic analyses of different taxa, e.g., individuals, populations, or species, are basically about estimating relative degrees of consanguinity. Put simply, the higher the proportion of shared traits/characters between two taxa, the higher degree of relatedness or, in the case of nucleotide sequences, the more mutations (i.e., differences in the nucleotide sequence) at the same locus separating two different taxa, the less related.
In principle, two kinds of mutations are observed in nucleotide sequences: single nucleotide substitutions or insertions/deletions of one or more nucleotides.
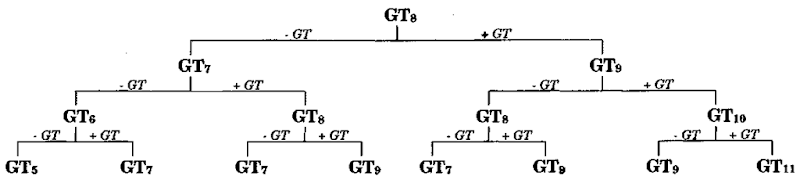
The latter kind of mutation is common at microsatellite loci. The most commonly analyzed microsatellite loci are dinu-cleotide repeats (e.g., GT), which are more common than tri-and tetranucleotide microsatellite loci in the mammalian genome. Most sequence changes at microsatellite loci consist of additions or deletions of one or more repeats. This kind of mutation is likely due to single-strand slippage, which subsequently results in misalignment during DNA replication. This mode of mutation is termed a stepwise mutation model (Fig. 2). Mutation rates at microsatellite loci are often high and have been estimated at 10-4-10~5. which is severalfold higher than that observed for single nucleotide substitutions. The high mutation rate typically yields multiple alleles at each locus and consequently high levels of heterozygosity. Microsatellite loci are thus well suited as genetic markers in the estimation of close relationships, such as parent-offspring relations. In contrast, microsatellite loci are less well suited to estimate more distant relationships due to high levels of homoplasy generated bv the high rate and mode of mutation (insertion/deletion of repeats). Alleles at a microsatellite locus will differ solely by the number of repeats, and two copies of the same allele (i.e., the same number of repeats) may be allozygous or autozygous (Fig. 2). This aspect has to be taken into account during data analysis, and several estimators of genetic divergence have been developed specifically for microsatellite loci. However, the stepwise mutation mode introduces additional variance in the estimation of genetic divergence, which in turn reduces the precision. While the probability of homoplasy is low among closely related individuals, such as members of the same population, it increases with genetic divergence and thus poses more of a problem at distant relationships. Other mutational constraints appear to occur at microsatellite loci as well, such as a limit on the number of repeats and rare niultirepeat mutations, both which affect the feasibility of microsatellite loci to estimate distant evolutionary relationships, such as divergent populations or different species.
The rate of single nucleotide substitutions is typically severalfold lower than that of single-strand slippage at microsatellite loci. The lower mutation rate implies that single nucleotide substitutions are less prone to homoplasy and thus in many ways are better suited than microsatellite loci to estimate more distant evolutionary relationships. However, the rate of single nucleotide substitutions differs among and within loci due to varying (often unknown) selective pressures. An example is codons in exons. In most cases a single amino acid is encoded by at least four different codons. The different codon sequences encoding for the same amino acid typically differ at the third position, at times on the first, and only rarely at the second codon position. Hence, nucleotide substitutions at the third position are usually synonymous and not subject to selective constraints. In contrast, the majority of nucleotide substitutions at the first and second codon positions are nonsynonyinous. The selective constraints are thus higher at the first and second codon positions, and the substitution rate is usually lower than that of the third codon position. Because of the different selective pressures relative to codon position, phylogenetic analyses usually stratify nucleotide sequence data according to codon position. There are, however, multiple exceptions to this rule of thumb.
In mammals the vast majority of the genome does not encode for proteins and is thus presumably under little or no selection pressure. However, the large variations in mutation rates among such noncoding DNA sequences indicate the existence of selective constraints acting on these DNA sequences as well. Possible explanations are aspects such as chromosome pairing during meiosis, replication and transcription rates, and chromosomal stability.
Figure 2 The stepwise mutation mode at microsatellite loci. +/— (GT) denotes a mutation by single-strand slippage, i.e., addition or deletion of a single GT repeat unit.
A prerequisite for the estimation of the relative degree of genetic divergence among taxa is a model of the underlying evolutionary mechanisms. One important assumption in most evolutionary models is the absence of homoplasy. The commonly employed infinite-site mutation model assumes that mutations always occur at a new site in the nucleotide sequence. The infinite-allele model differs slightly in that multiple mutations at the same position can occur, but no allozygous alleles have identical nucleotide sequences. The consequence of either model is that identical nucleotide sequences all are assumed to be autozygous. While these idealized models probably are applicable to closely related taxa, multiple mutations do occur at the same position, especially at fast evolving nucleotide sequences, such as the commonly analyzed mitochondrial control region.
The earlier mentioned variance in mutation rates among loci is in fact an advantage as it enables the researcher to pick loci with mutation rates appropriate for the level of divergence under study. Usually the goal is to uncover sufficient amounts of variation to facilitate accurate estimations, while keeping the amount of homoplasy as low as possible.
Mammalian cells, like all eucaryotic cells, contain two different genomes; the cell nucleus harbors a paternal and maternal set of chromosomes, and the mitochondria, in the cell cytoplasm, possess a small genome, a circular DNA molecule of approximately 16,500 nucleotides in length in cetaceans and pinnipeds. During formation of the zygote, the sperm cells do not seem to contribute any mitochondria in mammals, although rare cases of paternal leakage of mitochondrial DNA have been reported. Thus in principle and for all practical purposes, the offspring inherits only the maternal mitochondrial genome.
III. Analyses of Individuals
In the case of marine mammals, genetic methods have been applied to identify individuals and parent-offspring relations as well as full siblings for a number of different purposes.
Identifying marine mammals by traditional tagging methods is often not feasible in most species. In many instances, marine mammals are simply too large, have too wide ranges, and live in a too dense medium to make traditional tagging practical. Tag attachments are usually relatively short-lived, in part because of the significant drag caused by the water unless attached to solid structures, such as the tusk of a male narwhal. While individual identification from natural markings has been applied successfully to a number of marine mammal species, this approach is limited to species with sufficient levels of natural variation among individuals.
In comparison, individuals from most species can be identified by “genetic fingerprinting,” even species with much reduced levels of genetic variation, such as northern elephant seals, Mirounga angustirostris. Palsb0ll and co-workers (1997) set out to verify if “genetic tagging” was feasible for a wide-ranging cetacean species. Their study included 3068 skin biopsy samples collected over a period of 8 years (from 1988 to 1995) from humpback whales, Megaptera novaeangliae, across the North Atlantic. Each humpback whale was identified by its composite genotype collected from six hypervari-able microsatellite loci. The main issue in individual identification from a genetic profile is the probability of identity. The probability of identity is estimated readily for all degrees of consanguinity, ranging from unrelated individuals to parent-offspring pairs, from the population allele frequencies and decreases with the number of loci genotyped. The difficulty lies in determining the proportion of each kind of relationship among the collected samples, which in turn determines the expected number of individuals that have identical genetic profiles by chance. While the probability of identity is positively correlated with the degree of consanguinity, the proportion of pairs of a specific degree of relation decreases with consanguinity. In the case of the humpback whale study mentioned earlier, the probability of identity and expected numbers of different individuals with identical composite genotypes were estimated for unrelated individuals only, first for each maternally related feeding aggregation and subsequently for the entire population. The expected number of pairs of different individuals with identical genetic profiles by chance in the total sample of 3068 samples was estimated at less than one. Consequently, skin biopsy samples with identical genetic profiles were inferred as originating from the same individual. In total, 698 such samples with duplicate genetic profiles were detected. In a few cases, samples had been collected from the same individual humpback whale as far apart as 7500 km. The overall pattern of resightings within and among samphng areas was in agreement with data based on two decades of sighting records of individual humpback whales from their natural markings. The genetic “tags” were also used to estimate the abundance of humpback whales on the breeding grounds in the West Indies using mark-recapture techniques. Because the sex of each individual whale had been determined by genetic analysis as well, separate estimates of male and female abundance were calculated. Unexpectedly, the study yielded a significantly higher estimate of males at 4894 (95% confidence interval, 3374-7123) relative to that of females at 2804 (95% confidence interval, 1776^1463). The reason for this apparent underrepresentation of females on the breeding range (the sex ratio among calves and all whales on the feeding grounds has previously been estimated at 1:1) could not be resolved on the basis of data collected during the study. However, the authors suggested either spatial or temporal segregation among females as the source of the difference between the two abundance estimates.
An aspect of marine mammal biology where genetic methods are especially useful is the determination of parentage, e.g., to study breeding strategies and to assign individual reproductive fitness. Paternal reproductive success can be assessed in several ways, either by determination of specific parentage or by the level of paternal variation among the offspring. The former approach is relatively straightforward, as individuals that are related as parent and offspring will have at least one allele in common at each locus. However, as is the case for individual identification (see earlier discussion), two individuals that are not related as parent and offspring may also share at least one allele at each locus by chance. The probability that two individuals not related in parent-offspring manner share one or two alleles at each locus by chance decreases with the number and variability of loci genotyped. Hence, confident assignment of parentage requires that a relatively large number of variable loci are genotyped. In addition to a sufficient number of genetic markers, an adequate set of samples is required in order to ensure that parent and offspring pairs are contained among the samples collected. To date, only a few studies have attempted assignment of paternity in marine mammals, e.g., in gray seals, Halichoerus gri/pus, or harbor seals, Phoca vitulina, by analysis of either microsatellite loci or “multilocus” DNA fingerprinting as in the case of the northern elephant seal where genetic diversity is exceptionally low.
Hoelzel and co-workers compared the reproductive success of northern and southern, M. leonina. male elephant seals estimated as the proportion of pups fathered by the alpha male in his own harem. Previous behavioral observations indicated a higher level of competition for matings among male northern elephant seals compared to male southern elephant seals, leading to the hypothesis that northern elephant seal alpha males on average are less successful than their southern conspecifics. The genetic analysis corroborated this hypothesis, finding that southern elephant seal alpha males sired a significantly higher proportion of pups in their own harem than did northern elephant seal alpha males.
Multilocus DNA fingerprinting diiiers from microsatellite analysis mainly by the fact that the alleles from multiple loci are detected simultaneously. The simultaneous detection of multiple loci prevents the assignment of individual alleles to loci, which is why the degree ol relatedness usually is estimated from the proportion of bands shared between individuals. However, the relationship between the degree of band sharing and relatedness is not straigl it forward, which is why the degree of band sharing is usually calibrated with a sample of individuals of known relationship, i.e.. parent-offspring pairs.
Amos and co-workers (1993) employed multilocus fingerprinting as well as microsatellite loci to study the pod structure of long-finned pilot whales. Globicephala melas. Long-finned pilot whales are found in groups known as pods. Pilot whale pods appear to consist of mature animals as well as immature animals, presumably calves of the mature females. However, genetic analyses revealed that adult males within a pod were also closely related to mature females in the same pod. indicating that males stay within their natal pod. even after they become mature. Genetic analyses further revealed that mature males had not sired the calves in their own pod. Curiously, calves of the same cohort in a pod shared paternal alleles, indicating that a single or few closely related males sired calves of the same age. The authors proposed that mature males leave their natal pod briefly and mate with receptive females when pods meet during the breeding season. This hypothesis would explain why no males were found to have sired calves in their own pod, as well as the observation of few paternal alleles among calves belonging to the same cohort within a pod. Mature males of different ages within a pod would then also be maternally related.
Individual-based analyses like examples just given have the potential to address new issues with genetic methods that previously were not feasible. Traditional population genetic analyses (see later) yield evolutionary estimates of genetic divergence and may thus be of limited relevance to contemporary management and conservation issues. However, identifying individuals and parent-offspring relations provides a “real time” insight into population structure and dispersal at a time scale relevant to management and conservation purposes.
IV. Analyses of Populations
A large number of genetic studies of marine mammals have been undertaken lor the purpose of identifying population structure and mechanisms of intraspecific evolution. In practical terms the aim is to determine if individuals belonging to the same partition are more closely related to each other than with individuals from other partitions, which is expected if partitions represent different t axon omit units (e.g., pods, population, or species). In numerical terms, this objective translates into estimation of the degree of genetic heterogeneity among subpop-ulations. The degree of genetic heterogeneity among subpopulations is traditionally estimated as the relative increase in homozygosity due to population subdivision, e.g., Wright’s F statistics. The increase in homozygosity due to population structure is a product of random genetic drift. Random genetic drift denotes the oscillation in allele frequencies resulting from sampling each new generation from the parental generation. If we assume that mating is random within each subpopulation with respect to the locus under study (which is likely to be the case in most instances) and the absence of any selection, the offspring generation can then be viewed as a random sample ol the parental alleles. As with any random sampling process, such sampling is subject to stochastic variation, i.e.. alleles are not resampled in exactly the same proportions as those found in the parental generation and the allele frequencies will thus oscillate between generations. The long-term consequence of random genetic drift in a finite-sized subpopulation is that all but one allele will be lost from the subpopulation, in the absence of introduction of new alleles by gene flow and mutation. In other words, clue to random genetic drift, alleles are lost from a subpopulation (increasing the homozygosity) at a rate depending on the rate of introduction of new alleles by either mutation and/or gene flow from other subpopulations. Because the process is random, it follows that different alleles will in/ decrease in frequency due to random genetic drift in different subpopulations. Overall the effect of random genetic drift is that we find more homozygotes among the sampled individuals (collected from more than one sub-population) than expected from the overall allele frequencies if our sample contains individuals from a single random mating subpopulation. Gene flow will homogenize the allele frequencies among sub-populations by transferring alleles from one subpopulation to others. If there are no major fluctuations in effective population size, gene flow, or mutation rates, an equilibrium state is reached where the divergence in allele frequencies caused by random genetic drift and mutation is equivalent to the rate of homogenization due to gene flow. Even very low levels of gene flow (e.g.. 10 individuals per generation) among subpopulations will homogenize allele frequencies among subpopulations to an extent that no effect of random genetic drift and mutation can be detected. Neither the mutation rate nor the effective population size is usually known in natural populations. For instance, two populations may have a similar level of genetic variation (e.g., estimated as the heterozygosity) but differ in terms of population sizes and mutation rates. For instance, the degree of heterozygosity estimated among samples collected from a small population at loci with high mutation rates may be similar to that estimated from a large population at loci with low mutation rates. As the level of genetic variation depends on the combination of effective population size and mutation rate (and these are typically unknown), it is common to simply combine both in the composite parameter 0 = 4A’,.|x, where Ne denotes the effective population size and fx the mutation rate. The advantage of this approach is that the composite parameter 0 can be estimated from population genetic data, i.e., from the number of alleles, heterozygosity, polymorphic nucleotide positions, and the variance in allele size (for microsatellite loci). Comparisons of estimates of 0 can be used to draw inferences regarding differences in mutation rates among loci within single populations or differences in effective population size among populations as well as estimates of genetic divergence.
As mentioned earlier, many population genetic studies of marine mammals have employed analysis of microsatellite loci. In addition, the nucleotide sequence of the maternally inherited mitochondrial control region is usually determined as well. The mitochondrial control region constitutes the only major noncoding region of the mitochondrial genome, with mutation rates well above the remainder of the mitochondrial genome. Usually the sequence of the first 200-500 nucleotides in the mitochondrial region is determined, which constitutes the most variable part of the mitochondrial control region. Because the mitochondrial genome is maternally inherited, any results from this locus estimate only the degree of maternal relation among samples. Most microsatellite loci, however, are of autosomal origin and thus inherited in a Mendelian manner.
The different mode of transmission of the mitochondrial and nuclear genome implies that each may reflect a different evolutionary relationship for the same set of samples. Palumbi and Baker investigated this aspect in 1994 in a study of humpback whales. In addition to mitochondrial control region sequences, the study also included data collected from the first intron in the nuclear protein-encoding locus actin. A phylogenetic analysis of actin intron I allele nucleotide sequences revealed the existence of two main evolutionary lineages with no apparent geographic affinities. The two lineages could be distinguished by digestion with the restriction endonuclease Mnl\ basically defining a system of two alleles. This two-allele system was subsequently employed in the analysis of samples collected off Hawaii and western Mexico, both winter breeding grounds for eastern North Pacific humpback whales. While the distribution of mitochondrial control region alleles was highly heterogeneous between the same two population samples (the Hawaiian sample being almost monomorphic), no significant level of heterogeneity was detected in the distribution of the two actin intron I alleles. These “contrasting” results, i.e., little/no gene flow at the mitochondrial locus but indications of high levels of gene flow at the nuclear actin intron I locus, were interpreted as the result of male-mediated gene flow, different rates of random genetic drift at each of the two genomes, or a combination of both. However, a subsequent study by Baker and co-workers also revealed significant levels of heterogeneity at nuclear loci (mainly microsatellite loci) among humpback whale samples collected off California and Alaska, which winter off Mexico and Hawaii, respectively. The simplest explanation for the seemingly discrepant outcome of the two just-mentioned studies is likely an increase in statistical power due to larger sample sizes and the inclusion of additional nuclear loci in the analysis (actin intron I as well as four microsatellite loci). However, the results do not rule out the possibility of some contribution from male-biased gene flow to the level of heterogeneity. More work is necessary before any affirmative conclusions can be reached.
The issue mentioned earlier, i.e., different degrees of male and female gene flow, is highly relevant when studying marine mammals. This has been clearly demonstrated in several population genetic analyses of species such as the North Atlantic humpback whales as well as northern right whales, Eubalaena glacialis, and belugas, Delphinapterus leucas. Specifically, North Atlantic humpback whales summer at several high-latitude feeding grounds off the eastern sea border of North America, west Greenland, Iceland, Jan Mayen and Bear Island in the Barents Sea. Humpback whales from these distinct feeding grounds all appear to congregate on common winter grounds in the West Indies. The winter constitutes the breeding and mating season. Calves are bom during the winter and follow the mother during the spring migration to a high-latitude feeding ground and later on the autumn migration back to the West Indies. At the end of their first year the calves separate from their mother. The calf will, however, continue to migrate back to the same high-latitude feeding ground in subsequent summers to which it went with its mother during the first summer. The population genetic consequence of this maternally directed migration pattern is that North Atlantic humpback whale summer feeding grounds can be viewed as a single panmictic population with respect to nuclear loci, but structured in terms of mitochondrial loci. The latter is due to the maternal transmission of the mitochondrial genome in combination with the maternally directed site fidelity to the high-latitude summer feeding grounds. Nuclear alleles are exchanged when humpback whales from different summer feeding grounds mate in the West Indies. However, the calves only inherit their maternal mitochondrial genome and thus there is in principle no exchange of mitochondrial DNA among summer feeding grounds if calves keep returning to their maternal high-latitude summer feeding ground. Several population genetic studies have analyzed North Atlantic humpback whales and, in conclusion, found what was expected from the explanation just described. However, low levels of heterogeneity have also been detected at nuclear loci when comparing western and eastern North Atlantic high-latitude summer feeding grounds, indicating that some eastern North Atlantic humpback whales may winter and breed elsewhere than in the West Indies.
On a more detailed spatial scale, Hoelzel and co-workers determined the genotype at multiple microsatellite loci and the nucleotide sequence in the variable part of the mitochondrial control region in samples collected from pods of killer whale, Orca orcinus, observed in Pnget Sound in the northeastern Pacific. Two kinds of killer whale pods are found in Puget Sound: resident and transient. The latter pods spend only part of the year in Puget Sound. While the resident pods seem to feed almost exclusively on fish, the diet of transient pods is mainly composed of marine mammals. The two kinds of pods also differ in the average number of individuals and vocalizations. Genetic analysis revealed significant levels of heterogeneity between resident and transient killer whales not onlv at the mitochondrial locus but at nuclear loci as well. This result was inferred as evidence of restricted gene flow between two different kinds of foraging specialists, and in fact it might be that this feeding specialization drives the genetic divergence between the two sympatric groups of killer whales.
All the just-mentioned examples assume the absence of selection, but one could well envision natural selection affecting the degree and distribution of genetic variation among and within subpopulations.
One such possibility is the sperm whale, Physeter macrocephalus, among which very low levels of variation have been detected in the mitochondrial control region on a worldwide scale. This observation prompted Whitehead to propose selectively advantageous cultural transmission in matrilineal whale species as the cause of the low levels of variation at maternally inherited mitochondrial loci. The basic principle proposed by Whitehead is that long-term association between females and their offspring facilitate an efficient cultural transmission of behavioral traits, e.g., feeding behaviors. If a maternal lineage adopts more efficient behaviors that are selectively advantageous, which in turn increases that lineage’s reproductive success, such maternal lineage will eventually increase in proportion within the population. The model is similar to the genetic inheritance of selectively advantageous traits, i.e., natural selection, the only difference being that transmission across generations is cultural as opposed to inheritance. Because the mitochondrial genome is transmitted maternally, it will thus “hitchhike” along with the maternal cultural transmission of advantageous behavioral traits. The study reported low levels of genetic variation at mitochondrial loci in species which were classified as matrilineal by the author, i.e., species with pods, presumably consisting of females and their offspring, such as pilot whales (Glohicephala spp.) and sperm whales. In contrast, the nucleotide diversity was on average 10-fold higher in species classified in the study as nonmatri-lineal. Using computer simulations, the author demonstrated that the maternal cultural transmission of advantageous behavioral traits could indeed reduce the nucleotide sequence variation at mitochondrial loci significantly if the cultural transmission was efficient and the selective advantage was relatively high (—0.1). While there was no objection to the hypothesis that cultural transmission of advantageous behavioral traits might occur in cetaceans, others have pointed toward other evolutionary models, such as continued selection and fluctuating population sizes, as equally compatible with the observed data collected from sperm whales.
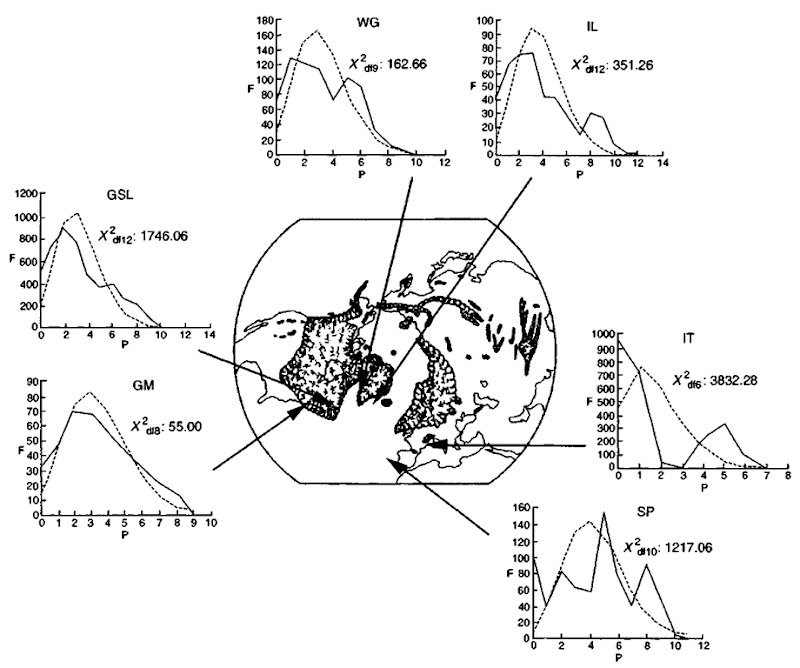
The environment inhabited by marine mammals is relatively devoid of physical barriers in comparison to the terres trial environment. In addition, many marine mammal species have wide ranges and thus there is a high potential for dispersal. Despite this, most genetic studies of marine mammals have detected population structure in the distribution of genetic-variation within as well as between ocean basins. The lack of physical barriers to dispersal indicates that intrinsic factors may play a role in generating population structure, such as foraging specialization and maternally directed site fidelity. Even for species where no obvious behaviors limiting dispersal have been observed, population genetic structure was detected, such as in the case of polar bears, JJrsus maritimus, and fin whales, Balaenoptera physalus. In these two instances, it appears that the availability of prey is, at least in part, responsible for generating population genetic structure. In the case of polar bears, Paetkau and co-workers (1999) analyzed 16 different microsatellite loci in a total of 473 polar bears collected from all areas of the Arctic. The study detected a pattern of genetic divergence among subpopulations that was consistent with the distribution of active annual sea ice, which in turn relates to the abundance of ringed seals, which is their main prey. The study of North Atlantic and Mediterranean Sea fin whales by Berube (1998) was based on analyses of mitochondrial control region sequences as well as six microsatellite loci in each of 309 specimens. The population structure revealed by the genetic analyses was consistent with an isolation-by-distance model, which could be explained by a distribution, described as a “patchy-continuuin” previously suggested by Sergeant and based on the overall distribution of prey. Interestingly, the fin whale study also revealed the possible effect of major geological events, in this case glaciation, on the present-day levels and distribution of genetic variation. The frequency distribution of mitochondrial nucleotide sequences suggested that the fin whale population in the western North Atlantic had undergone rapid expansion in abundance most probably from a small postglacial founder population (Fig. 3).
V. Analyses of Interspecific Relationships
A well-founded phylogenetic description of marine mammals is fundamental to our understanding of the unique evolution and adaptations observed in this group of mammals. Phylogenetic studies have been conducted at several levels, e.g., among cetaceans as well as at higher levels, such as the relation of cetaceans to ungulates.
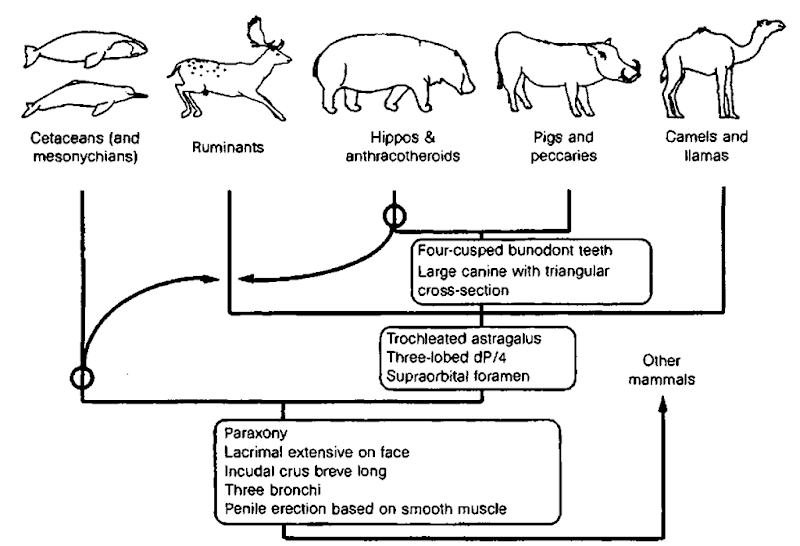
The latter question has attracted much attention as molecular data are emerging complementing earlier morphological estimates of the phylogenetic affinities of marine mammals. Results emerging from the molecular data are, at the moment, inconsistent with the morphological findings as well as among the different molecular data sets themselves with regard to the branching order in several parts of the evolutionary tree. There are multiple possible explanations for such incongruence, such as incomplete taxonomic sampling, inadequate model of change (molecular and morphological), insufficient choice and number of outgroups, as well as incomplete fossil records. As mentioned earlier, the level of homoplasy increases with genetic divergence, which complicates the interpretation of nucleotide sequence data. Instead of DNA nucleotide sequences, the more common sort of data employed in phylogenetic analyses, Shi-mamura and co-workers mapped the presence or absence of retroposons, termed short interspersed elements (SINEs), at different locations in a number of ungulate and cetacean species. SINEs are in many ways thought to be ideal phylogenetic characters as they presumably are inserted into the host genome in a random and irreversible manner, i.e., a very simple mutation model devoid of many of the problems, such as homoplasy, codon position, transition/transversions ratio, and the like, which introduce variability in analyses of single nucleotide substitutions. The SINE-based study found support for the notion that Artiodactyla is a paraphyletic group in that cetaceans did not constitute a sister group but originate within Artiodactyla (Fig. 4). Earlier studies based on a sequence analysis of nuclear loci encoding milk proteins by Gatesy (1998) also arrived at the same conclusion, i.e., that artiodactyls are a paraphyletic group, also from the position of the cetacean branch. The paraphyly of Artiodactyla was subsequently supported in a comprehensive phylogenetic estimation conducted by Gatesy involving data from several nuclear and mitochondrial loci. Given the highly specialized cetacean morphology, comparisons of moqjhological characters with terrestrial mammals are not a trivial matter either and may in part account for the observed discrepancies between the morphological and molecular approaches.
Figure 3 Indication of postglacial expansions on western North Atlantic fin whale, Balaenoptera physalus, popidat ions from genetic data. Observed (solid line) and expected (dashed line) frequency distributions of pairwise differences among mitochondrial control region nucleotide sequences in North Atlantic fin whale populations under a model of exponential expansion (see text for details). A close match between the observed and the expected distribution suggests that the sa mples were obtained from an exponential expanding population. The marked areas on the map of the Northern Hemisphere indicate the presence of solid ice sheets during the last Pleistocene glaciation.
Figure 4 Changes to the traditional artiodactyl phylogeny suggested by the findings of Shimamura et al. (1997). See text for details.
A perhaps more controversial study is that of Milinkovitch and co-workers (1993) who estimated the phylogenetic position of the sperm whales within Cetacea from mitochondrial nucleotide sequences. Conventional taxonomy based on morphological characters places this distinct and old lineage of cetaceans among the odontocetes, as sperm whales share many morphological characters with other odontocetes, the presence of teeth and echolocation being the most obvious traits. In contrast, the study by Milinkovitch and co-workers found that sperm whales were significantly more closely related to the baleen whales than to the remainder of the odontocetes. The result of this study has since been the subject of numerous additional analyses and reanalyses and, in many ways, has become a case study of phylogenetic estimation. These additional analyses have showed that estimation of taxonomic relationships from nucleotide sequences is sensitive to aspects such as choice of outgroups, taxonomic sampling, sequence alignment, and long branches. Subsequent analyses based on nuclear and mitochondrial loci by Gatesy showed a strong support among nuclear genes for the traditional odontocete affinity of the sperm whales and less strong support for the alternate view suggested by the mitochondrial nucleotide sequences.
In both of the instances just described, the lack of congruence among the different approaches and loci demonstrates that our understanding is still far from satisfactory and that additional work is necessary before we have a more thorough and definitive understanding of the evolution of this highly specialized group of mammals and the underlying molecular mechanisms employed in our inferences.