I. Patterns of Evolution
Fossils show that cetaceans arose from terrestrial ancestors more than 50 million (M) years ago. They have evolved to become the dominant group of marine mammals in terms of both taxonomic and ecological diversity and geographic range. Fossils provide the only direct evidence of cetacean evolution and extinction, revealing many changing patterns over time, but rarely details of rates, modes, and mechanisms of ancestor-to-descendant transitions. Structural, genetic, and ecological patterns in living species also elucidate how Cetacea have evolved. At the species level, evolutionary processes include natural selection, sexual selection, coevolu-tion, founder effects, vicariance, and hybridization. At larger scales, the distribution and abundance of global food resources have also played major roles in evolution, for there are strong links between changes in ocean structure and ocean ecosystems on one hand and structural/ecological patterns among Cetacea on the odier.
II. Paleoecology and Evolution
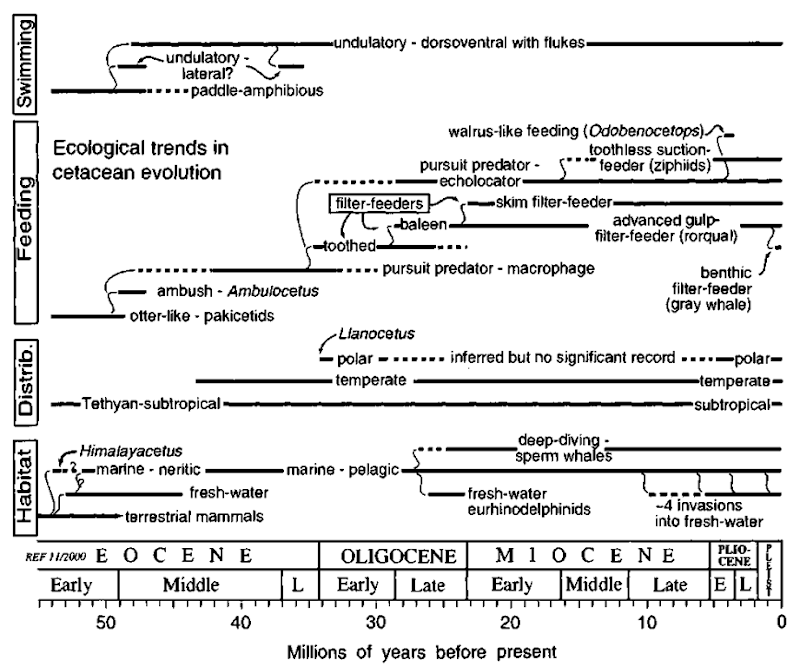
Ecology encompasses the sum of interactions that species have with their environment, biological, physical, and chemical, whereas paleoecology explores such relationships for the past. At the scale of geological time, evolution and paleoecology are inextricably linked, for evolution occurs through natural selection and adaptation to the environment. An appreciation of paleoecology thus helps understand the history of Cetacea. Several ecological factors have been considered repeatedly in accounts of cetacean evolution (Fig. 1), including feeding and predator/prey interactions, migration, thermal adaptations, and habitat shifts.
For any ancient species, paleoecology can be inferred several ways. A common approach, termed “taxonomic uniformi-tarianism,” assumes that a fossil species had similar ecological strategies to its closest living relatives. Thus, fossil sperm whales presumably were deep divers, and fossil mysticetes were filter feeders. Fossils show that all cetacean lineages have evolved through a long-term change in structure. If such evolution is caused by ongoing adaptation to changing environments, then even within a lineage, ecological strategies will change. For these reasons, taxonomic uniformitarianism must be supported by other methods.
In groups that lack close living relatives, ancient ecologies may be inferred by analogy. The bizarre extinct Peruvian Odobenocetops species, or walrus whales, have no structural equivalents among living Cetacea, but their jaw form was remarkably similar to that of the unrelated living walrus, Odobe-nus rosmarus. A comparable style of suction feeding on molluscs is inferred. This approach to paleoecology can be expanded by studying functional morphology, which includes reconstructing soft tissues onto fossil bones using known conservative patterns of muscle, nerve, and vessel to bone. Finally, geological evidence (sediment patterns) and geochemical signals (isotopes) can identify the ancient environments in which cetaceans lived, sometimes indicating dramatic or novel ancient ecologies.
Changes in structure and diversity of fossils reveal major functional, ecological, and taxonomic shifts in cetacean history. In particular, these involve an Eocene radiation of archaeocetes and an Oligocene radiation of Neoceti. Ecological shifts in the later Miocene radiation of some odontocetes and mysticetes are less clear.
Figure 1 Summary of inferred ecological strategies important in cetacean evolutionary history. Paleoecology is inferred mainly using taxonomic uniformitarianism (see text). Only selected taxa are shown.
III. Major Phases of Cetacean Evolution
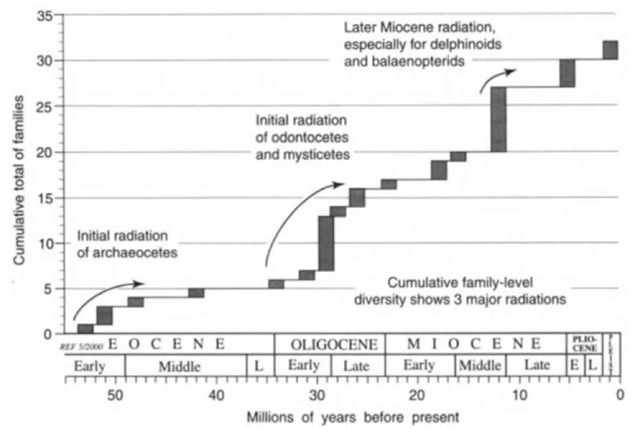
The fossil record reveals three major radiations in cetacean history (Fig. 2). First, cetaceans diversified in near-tropical shallow waters of the Tethys seaway between India and Asia about 45-53 million years before the present, (Ma), spreading into midtemperate waters by 40 Ma. Presumably this early phase of evolution was influenced by local marine productivity. Up to 6 species in three families of Archaeoceti (stem or basal Cetacea) were present at some times, and >35 species have been reported for the interval 35-53 Ma. This initial radiation of archaeocetes involved shifts from riverine and nearshore marine settings to fully oceanic habits, linked with changes in, for example, locomotory and hearing mechanisms. In terms of structural variation (and, by inference, ecology), these early cetaceans were comparable with living mysticetes and did not show the wide disparity seen among living odontocetes. Ar-chaeocete diversity dropped late in Eocene times, foreshadowing the rise of Neoceti.
The second major radiation, involving the Neoceti or crown group Cetacea, occurred early in the Oligocene. Echolocat-ing odontocetes and filter-feeding mysticetes arose from Late Eocene (~35-36 Ma) archaeocetes, which neither echolo-cated nor filter-fed, and diversified rapidly in about 5 M years. (No certain archaeocetes are known from the Oligocene.) Concurrent events included the final breakup of Gondwanaland, opening of the Southern Ocean, cooling and increased tropics-to-polar temperature gradients, and changes in ocean ecosystems and productivity. Probably, cetaceans radiated in direct response to new ecological opportunities in rapidly changing oceans. By the Late Oligocene interval (23.5-~29 Ma), there were 13+ families and probably >50 species, with some species ranging into polar and fresh waters.
Third, in about the middle of the Miocene (12-15 Ma), seven extant families of mysticete and odontocete appeared. Balaenopterid whales radiated to become the most speciose mysticetes, concurrent with a decline in archaic grades of mysticete (“cetotheres”). Delphinoids—dolphins and relatives—diversified dramatically, particularly the family Delphinidae, but also the Phocoenidae and Monodontidae. Two extinct delphi-noid families (Odobenocetopsidae, Albireonidae) appeared briefly. Some archaic cetacean groups, including the Eurhin-odelphinidae and Squalodontidae, went extinct at about this time, and the superfamily Platanistoidea (which includes the Squalodontidae) declined almost to extinction. Evolutionary patterns during the Quaternary ice ages (<2.5 Ma) are not clear because of a patchy fossil record. It has been suggested that many north-south species pairs evolved late in Quaternary times, but molecular studies point to older evolutionary events (see later).
IV. Processes: Mechanisms of Evolution
Darwin’s original evolutionary mechanism, natural selection, is the process that leads to the adaptation or “fine-tuning” through beneficial structure or behavior, resulting in differential reproductive success. Natural selection alone does not, however, explain the origins of novel structures. It is inescapable that the process occurs, although it is not the only one significant in evolution.
Figure 2 Cumulative graph of changes in family level diversity over time based on the cetacean fossil record. Three major radiations are apparent. Most of the earliest records for families are based on individual specimens with likely age errors of ±1 million years. With ongoing refinement of dating and the breakup of family level grades, more families are likely to be added in the future, some families of crown group Cetacea will be pushed morefinnly back into the Oligocene, and dating errors will be reduced.
Intra- or interspecific competition for limited resources, especially food, has long been viewed as fundamental in evolution. Many cetologists have suggested or implied that competition for food is important among species of baleen whales and between baleen whales and other plankton-eating vertebrates, with implications for the origins of the species involved. However, there is little direct evidence for this. The structure of the feeding complexes of baleen whales, mode (behavior) of feeding, and geographic distribution indicate that niche overlap and thus competition are limited between species. Perhaps previous competition among ancestral species led to taxonomic and ecological divergence seen today. Among fossils, platanistoid dolphins declined in diversity later in the Miocene, as the del-phinoids diversified about 12 Ma; this pattern of extinction in one group and radiation in another could indicate that delphi-noids outcompeted platanistoids. However, differences in skull structure, especially involving the jaws, imply different ecological requirements, and thus limited competition, between the two groups. Perhaps changing oceanic circulation and climate at about this time caused the extinction of platanistoids and allowed the radiation of delphinoids.
Darwin identified sexual selection as a mechanism in evolution. Here, one sex chooses a specific member of the other sex as a mating partner. Sexual selection might explain the origin of marked sexual dimorphism, particularly in structures involved in display, and has been linked with polygamous mating systems. Dimorphic and possibly sexually selected examples among male Cetacea include the large dorsal fin in Orci-nus orca, conspicuous mandibular teeth in many ziphiids, prominent foreheads in species of Hyperoodon and Globi-cephala, the genital hump in some species of Stenella, and the prominent tusks in Monodon monoceros and the extinct Odobenocetops leptodon. Perhaps the marked size dimorphism in some species also results from sexual selection; examples include male Physeter macrocephalus—with its grossly enlarged forehead—and female Balaenoptera species.
Hybridization is potentially important in the origin of new species, and cetacean hybrids are known, for example, in Balaenoptera, between Phocoenoides and Phocoena, and between several genera of Delphinidae. However, no convincing cases for evolution by hybridization have been identified in Cetacea.
Coevolution, especially involving mimicry, predator/prey, and host/parasite interactions, has an acknowledged role as an evolutionary process. For cetaceans, mimicry, which graphically illustrates natural selection, is seen in pygmy and dwarf sperm whales, Kogia spp.; these have a remarkably shark-like form complete with underslung jaw and pigmented false gill slit. Presumably, to look like a predator will lessen the chance of being preyed upon. Predation has other roles in evolution. The presence or absence of predators (such as the killer whale, Orcinus orca) has been used to explain species-specific distribution patterns, including the distinctive and presumably ancient migration patterns of mysticetes from polar to warmer waters for breeding.
Convergent evolution occurs when species show similarity not inherited from a common ancestor. Mimicry is a form of convergence. Among Cetacea, the “river dolphins,” long discussed as if they form a real group, encompass species in four different families and in two superfamilies. However, Platanista is convergent in its riverine habits, small body size, and long rostrum with the three delphinoid genera lnia, Pontoporia, and Lipotes. In the past, the now-extinct Eurhinodelphinidae included some riverine or lacustrine species, also with long rostra. For living species, the southern delphinid Cephalorhynchus hectori is similar in body form and some aspects of ecology and behavior to the unrelated porpoise Phocoena phocoena (Pho-coenidae). Unrelated groups have convergently reduced and lost teeth, as seen in Physeteridae, Ziphiidae, and some Delphinidae (e.g., Grampus).
In biology, there is great interest in the developmental mechanisms leading to rapid evolutionary change in structure. A widely discussed mechanism is heterochrony, which involves a change in timing or rate of development of structures relative to the equivalent processes in an ancestor. Thus, some features might arise when juvenile structures persist into adult stages (paedomorphosis), whereas others arise when structures develop “beyond” that of the ancestral adult stage (peramor-phosis). Either process could generate new structures. Apparent paedomorphic features in cetaceans include the shortened intertemporal region and longer vomer and mesorostral groove in Neoceti, the rounded cranium and persistent interparietal bone on the skull of many Delphinidae and Phocoenidae, and the down-turned rostrum and relatively symmetrical skull in Phocoenidae. Possible peramorphic features include extra body parts (e.g., increase in number of vertebrae, as in Dall’s porpoise, Phocoenoides dalli, or phalanges) generated through a delayed halt in development.
V. Geography and Evolution
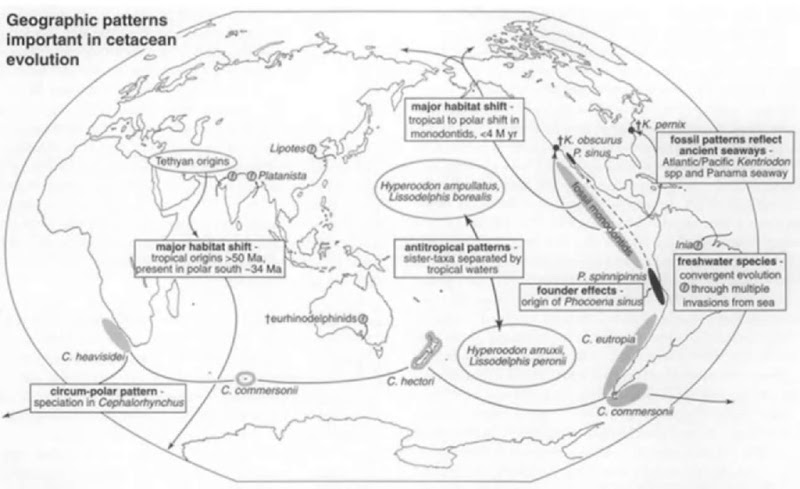
Darwin realized that speciation is often related to geography. The range of a species may be split by a change in physical habitat (namely, split by a vicariant event) or can be expanded by dispersal beyond normal limits. Populations that become geographically isolated through such events can diverge and, via allopatric speciation, may become new species. Most discussion of such ideas focuses on terrestrial habitats, but there are clear marine parallels. During 50+ M years of cetacean history, geographic changes have included the closure or opening of some straits and ocean basins, dramatic swings in continental shelf habitat area through sea level fluctuations, and major shifts in current systems, upwellings, and latitudinal water masses. Oceanic temperature regimes changed in parallel. This physical evolution of the oceans probably influenced cetacean evolution at many levels. The distributions of modern and fossil Cetacea indicate an important role for geography in evolution (Fig. 3).
Some living species have obvious northern and southern populations or closely related north-south species pairs, but do not occur in the tropics (Fig. 3). Such bipolar or antitropical distributions probably arose allopatrically when populations became isolated either side of the tropics through changing sea temperatures or current regimes, sometimes leading to speciation. Antitropical distributions are marked in populations of rorquals (Balaenoptera), species of right whales (Eubalaena), some beaked whales (Ziphiidae; species of Berardius, Hyperoodon, and Mesoplodon), porpoises (Phocoenidae), and dolphins (Delphinidae, e.g., Lissodelphis species).
Figure 3 Distribution patterns illustrating the possible role of geography in cetacean evolution. Patterns indicated include antitropical and circum-Antarctic distributions, allopatric species pairs between oceans, founder effects, convergent origins for various freshwater dolphins, habitat expansion in general for Cetacea (tropical origins, followed later by spread as far as the poles), and geologically recent changes in habitat. Only selected taxa are shown, Fossils are marked with a dagger.
Among delphinids, molecular studies reveal that six antitropical species of Lagenorhynchus split at varying times, not just during the geologically recent (<2.5 Ma) ice ages, and that these species represent two or more different lineages. At least one genus appears to have speciated around an ocean: four species in the small delphinid genus Cephalorhynchus occur around the circum-Antarctic Southern Ocean (Fig. 3). There is some fossil evidence for allopatric species pairs. Closely related species of the small archaic dolphin Kentriodon that occur in Miocene strata in California and Maryland perhaps evolved from an ancestor that ranged through the central American seaway before the uplift of Panama.
For porpoises, the endangered vaquita (Phocoena sinus), from the Gulf of Mexico, perhaps originated when a few of Burmeister’s porpoise, Phocoena spinipinnis, crossed the equator only tens of thousands of years ago (Fig. 3). The vaquita, with its limited distribution and low genetic variability, illustrates founder effects; a new population, for example, in an isolated region on the limits of a normal range, may be established by only a few original founders, which are not genetically representative of the original population.
Sea level fluctuations have changed the extent of continental shelf habitat dramatically, especially during cycles of glacia-tion and cooling over the last 2.5 M years. At peak glaciation, the sea level was >100 m lower than at present, leading to fragmentation or loss of shelf habitat for long intervals, along with colder conditions. This sort of habitat change could lead to extinctions. However, if species’ durations typically exceed 100,000 years, as is very likely, then most living cetaceans have survived several of these fluctuations. The record of Late Pliocene and Early Pleistocene Cetacea includes a few species from shallow-water habitats (the dolphin Parapontoporia, and cetothere Nannocetus), which are now extinct, perhaps as a result of Pleistocene climate changes and/or habitat loss.
Some cetacean groups show significant change in range, even in geologically recent times. The beluga and narwhal (family Monodontidae) are now restricted to cold north polar waters, but during wanner Pliocene times (~4 Ma), closely related extinct monodontids occurred far south in temperate to subtropical waters. Further evidence for changing geographic ranges comes from archaeocetes, which, for about 10 M years after their origin, were subtropical to tropical. In contrast, the maximum diversity for Cetacea is now in temperate waters.
Allopatric speciation results from lineage splitting, or clado-genesis. New species could also evolve by anagenesis, which involves transformation from an ancestral to descendant species without lineage splitting. Anagenetic change would produce a fossil record with several species in unbranched succession and might be expected in lineages containing geographically wide-ranged species. (Species with limited ranges seem more likely to experience allopatric speciation.) Among living Cetacea. the sperm whale Physeter macrocephalus and the killer whale represent genera with a single abundant and widely distributed species with no immediate relatives (sister species), and anagenesis might be considered in explaining their history.
Freshwater settings were important in the early transition of cetaceans from land to sea in the Tetliys (Fig. 1). Early archaeocetes were amphibious, with well-developed hindlimbs, and they include species from strata deposited in freshwaters. Freshwater habits for some archaeocetes are indicated by oxygen isotopes from teeth. Fossil Neoceti also show several rein-vasions of freshwaters. For example, one unnamed Late Oligocene species of Eurhinodelphinidae occurs in freshwater sediments of central Australia. The dolphin shows no obvious structural adaptations to a riverine or lacustrine habitat, but it is represented by several specimens of slightly differing geological age, indicating a significant long-term occupation of freshwaters. A single large ziphiid fossil is known from Miocene freshwater sediments of Kenya. All the living “river dolphins” arose from marine ancestors, some well known from fossils, which invaded freshwaters in Asia and South America in up to four separate events. Mysticetes seem never to have occupied river systems.
VI. Life History Strategies
In terms of life history traits, cetaceans appear to be K strategists. That is, compared with many other mammals, cetaceans are large animals that have slow reproductive rates, produce a single offspring, show significant parental cake of young, have long reproductive lives, and have relatively low mortality rates. This rather inflexible reproductive strategy has been linked, in an evolutionary sense, to the nutritional requirements of both the young and parents and thus to food availability. Physical disturbance of the environment and biological effects, such as predation, might be less important in cetacean evolution. The fossil record of cetaceans, which shows major evolutionary change linked to oceanic change, supports the idea that food resources have been fundamental in cetacean history.
VII. Taxonomic Duration
How long do species and genera persist? The fossil record shows that few, if any, species have ranges longer than one geological stage (a stage is a time unit of variable length, mostly 4-5 M years). For extinct Cetacea, the close-spaced succession of Eocene archaeocetes points to species’ durations of 1-2 M years. Living species can be traced back into the Pleistocene but not reliably longer, i.e., <2.5 Ma. Molecular studies provide estimated dates for separation of lineages of living species, including 1.9-3 and 3.8-9.6 M years for species of Lagenorhynchus and >5 M years for Balaenoptera musculus and B. physalus. Dates for lineage separation, however, are not necessarily the same as dates for species’ durations. For groups beyond the species’ level, some living genera have reliable fossil records back to the Early Pliocene or Late Miocene (~5 Ma), including Balaenoptera, Megaptera, Balaena, Eubalaena, Delphinus and Mesoplodon. Generally, though, generic ranges are uncertain because of inconsistent taxonomic concepts among paleontologists.
VIII. Rates of Evolution
Little is known about rates of evolution in Cetacea. The fossil record identifies phases of rapid radiation, and presumably rapid evolution, in the Early Oligocene and late Middle to early Late Miocene. Unlike the situation for some terrestrial mammals, the cetacean fossil record is not dense enough to reveal quantifiable change in structure over time. For living species, no speciation event has been dated reliably enough to clearly reveal evolutionary rate, but some species (vaquita, Phocoena sinus: gray whale, Eschrichtius robustus) probably evolved over tens, rather than hundreds, of thousands of years.
IX. Diversity and Disparity
Diversity is the number of species within a taxon such as a genus or family (effectively, an index of taxonomic richness), whereas disparity indicates the variation in structure or basic design within a taxon. Diversity is easy to assess, particularly now that advances in the philosophy and practice of classification have produced a widely accepted species level classification of living cetaceans, but the study of disparity is still developing.
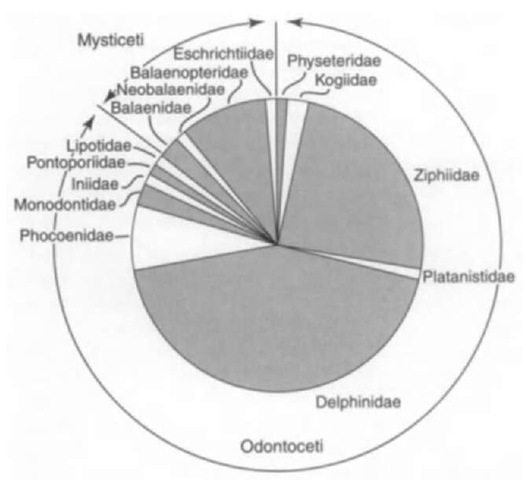
A comparison of the two living clades Odontoceti and Mysticeti reveals quite different patterns of diversity (Fig. 4) and disparity. Mysticetes include 12 species in three or four families. The Balaenopteridae is most speciose, with 8 species in two genera (Balaenoptera, 7; Megaptera, 1). Broadly speaking, species of Balaenoptera vary in size, distribution, and behavior, but species boundaries may be blurred. Species are distinguished mainly on aspects of the feeding apparatus (baleen size and spacing, size and shape of the upper jaw), and skeletal differences are rather minor. Thus, disparity appears low. The gray whale, Eschrichtius robustus, is structurally quite different (disparate) from other mysticetes and is placed in its own monotypic family. The gray whale probably arose geologically recently (~0.5 Ma?) among the balaenopterids and might be classified with that family despite its different form. In this case, disparity does not indicate taxonomic distance.
Odontocetes include 71-72 species in 10 families. With its 36 species, Delphinidae is the most diverse family of cetaceans, and disparity seems much higher than, for example, within Balaenopteridae. Among delphinids, there is a greater variation in body size and proportion, skull form, proportions of the feeding apparatus and teeth, and distribution of air sinuses in the skull base. Among beaked whales (Ziphiidae; 20 species), the genus Mesoplodon has 13-14 rather similar species in which only adult males are separated easily. Disparity here appears low and awaits explanation in terms of evolutionary ecology within the genus. Among other odontocetes, 4 species of small long-beaked “river dolphins” each represent a single family (Iniidae, Pontoporiidae, Lipotidae, and Platanistidae). Although these “river dolphins” are superficially similar externally, they are highly disparate in terms of skull form; this disparity supports the separation into 4 families. Of note, Iniidae, Ponto-poriidae, and Lipotidae are related closely to delphinoids, whereas Platanista gangetica is a highly disparate species in its own family (Platanistidae), in turn in the ancient and once diverse superfamily Platanistoidea, which originated about 30 M years ago. For Platanista, Platanistidae, and Platanistoidea, diversity is low (1 living species of Platanista, with no fossil record) and disparity is high.
Figure 4 Patterns of species’ diversity at the family level among living Cetacea. Traditionally, families are clusters of species and genera with a similar body plan; thus, families usefully reveal disparity at high levels within the Cetacea. Mysticetes are less speciose and less disparate than odontocetes; delphinids and then ziphiids are the most speciose and most disparate of the odontocetes. Species’ counts are derived from Rice (1998).
As a group, odontocetes are clearly quite disparate. Huge variation is seen in the skull, involving the shape, size, and sometimes basic construction of the feeding apparatus and teeth, facial region, including bony origin for nasofacial muscles implicated in echolocation, nasal passages, acoustic system, and air sinuses in the skull base. Disparity might be viewed as caused by reduced constraints on body form, allowing specialization in many different directions. This explanation for disparity, however, does not explain the ultimate origin of structural diversity.
X. Extinction
Extinction, the disappearance of lineages, is a fundamental complement to evolution. Extinction obviously occurs when the number of individuals and geographic range drops to nil. The fossil record reveals that extinction is inevitable and, for lineages, usually terminal; only a few species go extinct by evolving into descendants. Among different styles of extinction, there is no evidence that cetaceans have been involved in mass extinction comparable to that affecting dinosaurs. Taxonomic extinction (the disappearance of a real group or taxon) has occurred, as shown by the fossil record of such well-defined clades as the odontocetes Eurhinodelphinidae and Squalodontidae, and the Cetotherium group mysticetes. As noted earlier, environmental change might explain the demise of such groups through loss of habitat or food or of intolerance of cold. Most extinction has probably involved the piecemeal extinction of single species, but the cetacean fossil record is too patchy to expect pattern or cause to be clear. Species susceptible to extinction are those in low-diversity clades (e.g., one or two species in a genus, with no close relatives) occurring in geographically limited physical settings that are unstable over geological time; for Cetacea, this means particularly the “river dolphins.” Conversely, widely distributed oceanic species would seem resistant to extinction by other than human agencies.
Patterns of extinction beg a question that ecologists might consider unthinkable: are there vacant modern niches that formerly were occupied by Cetacea? For example, Platanistidae lived in shallow marine settings until about 10 Ma and now occur only in freshwaters, whereas species of Squalodontidae and Eurhinodelphinidae were widely distributed before their demise in the later Miocene. Judging from the functional complexes seen in the latter fossils, there are no modern equivalents to these groups. Functional studies are needed to see if some extant taxa may be the ecological equivalents of the extinct forms.