Insects are the only invertebrates to have developed the power of flight, and their wings are unique. Unlike those of birds and M bats, they are not modified limbs and so have no internal muscles; they consist predominantly of an extracellular material—cuticle. Typical wings nonetheless need to perform—semiautomatically—the complex movements and cyclic changes in attitude and shape associated with flapping flight, and these necessities strongly influence their structural design. The latter shows huge variation within the class Insecta, and this is made greater by the wide range of secondary functions that the wings have evolved to perform, sometimes instead of flight, sometimes in addition to it. While great progress has been made in interpreting wing structures in functional terms, many questions still remain to be answered.
ORIGIN
We have no fossil record of the earliest winged insects nor of the structures from which wings evolved. Majority opinion currently favors their origin from articulated lateral outgrowths—”exites”—of an obsolete basal segment of the legs, long since incorporated in the thoracic wall. Similar, although not homologous, structures are found on the coxae and abdominal segments of some apterygote insects. The articulated gill plates of juvenile Ephemeroptera may actually be wing homologues. Those of some fossil mayfly nymphs indeed resemble tiny wings, although this may simply reflect the fact that both are adapted for accelerating fluid.
The origin of flight is still controversial. Two conflicting theories both have significant support. The first, and more traditional, theory is supported by some experimental evidence and theoretical modeling and proposes that powered flight arose in tree-dwelling insects, through parachuting and gliding stages. These “protopterygotes” would have had winglets, probably movable, on most body segments. Selection would favor enlargement and improvement of winglets
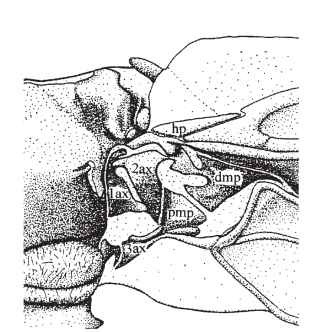
FIGURE 1 Forewing axilla and wing base of the desert locust, Schistocerca gregaria. 1ax, first axillary sclerite; 2ax, second axillary sclerite; 3ax, third axillary sclerite; dmp, distal median plate; hp, humeral plate; pmp, proximal median plate.
close to the center of mass of the body, and reduction and loss of the remainder, together with the evolution of an effective mechanism for flapping and twisting the wings. The second hypothesis suggests that flight arose in semiaquatic insects, initially using their winglets to skim on the surface film of water bodies, as do many modern adult Plecoptera and Ephemeroptera. Selection would favor enlargement and improvement of the thoracic winglets to the point at which they could generate sufficient upward force for the insects to leave the surface and fly.
STRUCTURE
Although some Carboniferous and Permian insects had short, apparently movable winglets on the first thoracic segment, the wings of modern insects are borne on the second and third segments only. They articulate with the back (tergum) and sides (pleura) of the thoracic segments via the axillae: complex, three-dimensional mechanisms of stiff and compliant cuticle through which the muscular forces of the thorax are transmitted to the wings and which control the relative movements of the wings’ basal components. The axillary structures vary greatly in detail, but those of most insects can be referred to a common plan of up to four so-called axillary sclerites and three other sclerotized plates, the humeral and the proximal and distal median plates, linked together by hinges and broader expanses of soft cuticle (Fig. 1). The axillary structures of Ephemeroptera and Odonata, orders that appear primitively incapable of folding their wings back over the abdomen, differ from the typical pattern of wing-folding (neopterous) insects and also from each other. Various attempts have been made to homologize these with the neopterous pattern, which is clearly influenced by the need to fold; but no consensus has been reached.
Orthodox wings themselves consist of cuticular membrane supported by, and continuous with, a framework of veins. Both veins and membrane are double structures, formed by juxtaposition of the cuticle of the dorsal and ventral sides as the wing increases in area, thins, and stiffens after the final molt. The veins are typically tubular and contain hemolymph, and major vein branches usually also carry branches of the tracheal system, and some include nerves from wing sensillae. The hemolymph circulates or moves tidally within the veins and serves to maintain the moisture content of the cuticle, which would otherwise become stiff and brittle. Little or no epidermal material appears to remain in the expanded, sclerotized adult wing.
The longitudinal veins run distally from the wing base and many branch as the wing broadens along the span. They are usually to some extent linked by crossveins. Together they form a supporting and conducting framework, which may be structurally very complex (Fig. 2C, 2F, 2I, and 2M) or relatively simple (Fig. 2D, 2E, 2J, 2K,and 2P).
Even the simplest wings are far more complex than conventional illustrations indicate. Most have considerable relief, which adds substantially to their rigidity. The primitive condition may well have been a radially pleated structure, with the longitudinal veins alternately occupying the ridges and troughs of the pleats. This is found in many early fossil insects and is still the case in Odonata and Ephemeroptera. Some pleating indeed persists in parts of most insect wings, although it may be absent elsewhere. Many wings show an arched, or cambered, cross section, which again enhances rigidity to bending and introduces other important properties, which will be discussed later.
Further features, often omitted from illustrations, but with great mechanical significance, are lines and bands of flexibility within the wing and the axilla. One can conveniently distinguish between flexion lines, whose primary function is the facilitation and control of wing deformations in flight, and fold lines, whose primary role is in folding the wings to and from the resting position. The distinction is not absolute: there may well be some limited deformation along flexion lines during wing folding and along fold lines in flight. Some other lines of flexible cuticle, for example, in the distal parts of some hymenopteran and dipteran wings, seem to fit neither category and are best interpreted as adaptations to allow the wing tip to crumple reversibly on impact with obstacles. The veins themselves vary considerably in diameter, wall thickness, and shape of their cross section, all factors that strongly influence their local resistance to bending and help determine the pattern of deformation in flight. Figure 3 shows some details of contrasting vein morphology. Many crossveins have a characteristic annulated form (Fig. 3F and 3G), a means of reconciling flexibility with the need to maintain an open section, as in flexible drinking straws and some hoses and flexible pipes. Elsewhere, crossveins can be stout and rigid and can even form high-relief angle brackets, as in the leading edge spar of dragonfly wings (Fig. 3D). Where longitudinal or crossveins are crossed by flexion lines and fold lines, they may be locally interrupted, thinned, or annulated.
The membrane itself can be thickened and sclerotized, either over substantial areas, as in the protective or semiprotective forew-ings of Coleoptera, Dermaptera, Heteroptera, Orthoptera, and Blattodea, or in more restricted lines or zones. In the latter case, it may not always be easy to distinguish the result from veins, especially since some true veins are not tubular, but are simply thickened, sclerotized grooves.
The texture of the membrane is also variable. Under the microscope, areas that undergo appreciable stretching in flight may appear
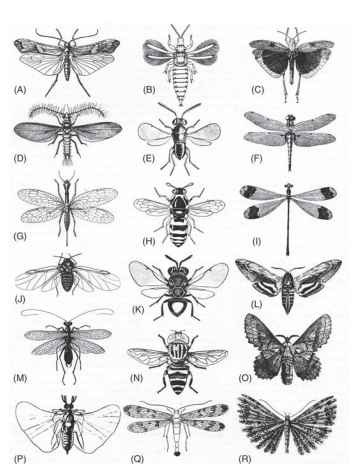
FIGURE 2 The variety of insect wings. The wing spans extend over two orders of magnitude, from ca. 1 mm in the thrips (B) to 100 mm in the damselfly (I). (A) Caddis (Trichoptera), Limnophilus rhombicus. (B) Thrips (Thysanoptera), Liothrips oleae. (C) Grasshopper (Orthoptera), Dissosteira carolina. (D) Male coccid (Homoptera), Icerya ppurchasi. (E) Parasitoid wasp (Hymenoptera), Coccophagus tschirchii. (F) Dragonfly (Odonata), Libellula quadrimaculata. (G) Snake fly (Megaloptera), Raphidia adanata. (H) Wasp (Hymenoptera), Celonites abbreviatus. (I) Damselfly (Odonata), Megaloprepus coerulatus. (J) Aphid (Homoptera), Eriosoma lanigerum. (K) Perilampid wasp (Hymenoptera), Perilampus chrysopae. (L) Hawk moth (Lepidoptera), Hyloicus ligustri. (M) Mantis (Mantodea), Mantoida brunneriana. (N) Hover fly (Diptera) Lathyrophthalmus quibquelineatus. (O) Lasiocampid moth (Lepidoptera), Gastropacha quercifolia. (P) Male stylopid (Strepsiptera), Eoxeonos laboulbenei. (Q) Scorpionfly (Mecoptera), Panorpa communis. (R) Plume moth (Lepidoptera), Orneodes cymodactyla.
crumpled like crepe paper, as in the hind wing fan of locusts, or as an expanse of hollow papillae, as in those of some Heteroptera. Microtrichia (small unarticulated hairs) are widespread and sometimes very dense, and the wing membrane of many insects, particularly among Holometabola, bears articulated hairs (e.g., Trichoptera) or scales (e.g., Lepidoptera, Psocoptera, mosquitoes). The scales are sometimes extraordinarily complex in structure, providing both insulation and color, including some spectacular physical colors. This complexity is particularly remarkable when one considers that each scale is produced by a single cell.
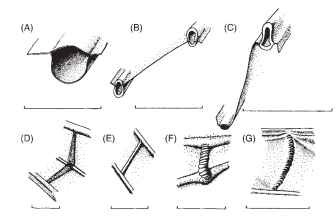
FIGURE 3 Details of veins. (A-C) Transverse sections of longitudinal veins. (A) Forewing subcosta of a butterfly, Papilio ruman-zowia (Lepidoptera). (B) Adjacent veins in the radial sector field of Calopteryx splendens (Odonata). (C) Ridge and trough veins from the hind wing fan of Schistocerca gregaria (Orthoptera). (D- G) Crossveins. (D) Angle-bracket-like crossveins from the leading edge spar of C. splendens (Odonata). (E) Crossvein from the remigium of the wing of C. splendens. (F) Flexible crossvein from Eristalis tenax (Diptera). (G) Flexible crossvein from the hind-wing fan of S. gregaria. Scale bars, 0.5mm.
The veins too may bear scales, hairs, and other ornamentation, including airflow- and vibration-sensitive hairs and sensillae that detect distortions of the cuticle in flight.
In several orders, the fore- and hind (meso- and metathoracic) wings are in physical contact during the wing stroke, the posterior part of the forewing overlying the anterior part of the hind and in many cases physically linked to it by a coupling mechanism. Some mechanisms may in fact provide sensory information about the relative positions of the wings rather than rigid mechanical coupling, but in Hymenoptera the hind wing leading edge bears a row of hooks that firmly clasp a fold of the forewing; in Heteroptera the hind wing edge is held by hairs in a groove in the forewing, and in many Homoptera the wings are held together by interlinking grooves.
HOMOLOGY AND TERMINOLOGY
Despite occasional claims to the contrary, there is no good evidence that wings arose more than once within the insects, and it is generally accepted that homologous wing characters should be recognizable throughout the class. In fact this is anything but straightforward. In the course of evolution, some veins have degenerated, or their identities have become obscured by fusion. Vein branches have been gained or lost, or have come to resemble crossveins. Linear, vein-like membrane thickenings have arisen and are readily mistaken for true veins. The homologies of flexion lines and fold lines, and the extent to which they can be used as landmarks in vein identification, are also far from obvious.
Vein Nomenclature
Unfortunately, several conflicting systems are in use. Two widely used schemes are illustrated here. Figure 4, adapted from Kukalova-Peck, shows all the longitudinal veins of any trace remains in extinct, as well as living, insects and is an attempt at an overall ground plan
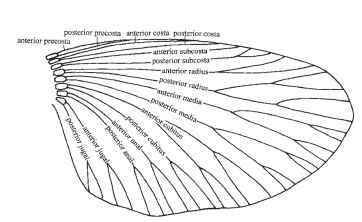
FIGURE 4 Wing vein nomenclature. The system of Kukalova-Peck.
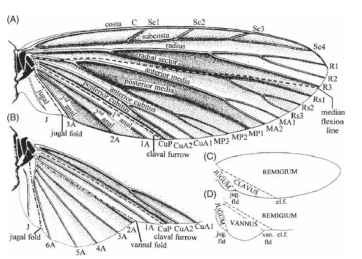
FIGURE 5 Wing nomenclature. A more traditional system, showing veins, some flexion and fold lines, and the main areas of the wing. (A, C) A forewing or a hind wing lacking an expanded ano-jugal area. (B, D) Cubital and anojugal region of hind wing with an expanded anojugal area.
for the winged insects. It proposes that veins were initially paired, the anterior member of each pair being convex and situated on a ridge of a pleated wing, and the posterior member being concave and situated in a trough. They are the anterior and posterior pre-costa, costa, subcosta, radius, media, cubitus, anal, and jugal. This scheme is consistent and logical and is increasingly widely followed. In most modern insects, however, several of the most anterior veins are obscure or absent, and many entomologists tend to ignore these, or indeed to question their existence, and to follow a scheme like that in Fig. 5. The figure shows (Fig. 5A) a diagrammatic wing, (Fig. 5B) the expanded anal fan (vannus) of some hind wings, and (Fig. 5C and 5D) the names in common use for specific regions of the wing. Kukalova-Peck’s precostal veins are omitted, as are her posterior costa and anterior subcosta. The anteriormost veins are hence the convex, unpaired costa and the concave subcosta. Kukalova-Peck’s posterior radius is called by the older name of radial sector. The number of anal veins is regarded as unfixed.
Both diagrams show most veins to be branched, as is often the case, but we do not in fact know what the primitive number of branches for any vein was. Crossveins are omitted from the diagrams. Where comparatively few are present in a wing, these are often named by the longitudinal veins that they connect, with the symbols for these veins given in lowercase type.
Regions of the Wing
In the wings of a neopterous (wing-folding) insect, distinct areas can be recognized, delimited by flexion lines or fold lines. The main, anterior area, between the leading edge and the claval furrow, is the remigium. In wings without an expanded anal fan, a narrower area between the claval furrow and the jugal fold is the clavus. The remaining, usually small area, which lies posterior to and inboard of the jugal fold, is the jugum. In the hind wings of many forms, the area between the claval furrow and the jugal fold is expanded into a broad anal fan, or vannus, which sometimes, notably in Orthoptera, Phasmida, and Blattodea, has fold lines within it and folds into a pleated fan.
Flexion Lines and Fold Lines
Flexion lines and fold lines have received comparatively little attention and indeed are often omitted from diagrams. In neopterous insects, one flexion line and one fold line are particularly widespread. The claval furrow is a flexion line that typically lies posteriorly to the posterior cubitus and allows the remigium to hinge upward or downward relative to the clavus. The jugal fold is continuous with the basal hinge of the wing and is involved in both flapping and folding.
Two other flexion lines are common, but their positions relative to other wing landmarks are not constant, and they may have arisen independently several times. The median, or remigial, flexion line runs longitudinally from the wing base, often between the radial and median vein systems. It allows the profile of the wing to be altered in flight and may influence its resistance to transverse bending. Transverse flexion lines, by contrast, facilitate ventral bending across the span. A transverse flexion line occurs in several orders, crossing the wing along a curved or irregular, sometimes oblique, path from the costa to the posterior margin.
DIVERSITY
The wings of insects are very diverse indeed—far more so than those of birds or bats. They vary in proportions; the relative sizes of fore- and hind wings; relief, texture, and ornamentation; thickness; venational richness; the details of veins, fold lines, and flexion lines; and the extent and manner in which they are coupled in flight. Figure 2 illustrates some of this diversity.
It is widely assumed, although without direct evidence, that fore- and hind wings were originally similar in size and shape. This is true today of zygopterous Odonata (Fig. 2I); of many Neuroptera, Megaloptera (Fig. 2G), Mecoptera (Fig. 2Q), Trichoptera, and primitive Lepidoptera; of Thysanoptera (Fig. 2B), Embioptera, most Isoptera, and a few Plecoptera (Chloroperlidae). In the last two orders at least the condition is clearly secondary. Some Permian fossil Ephemeroptera, Psocoptera, and Hemiptera also had similar fore-and hind wings, as did some members of several extinct orders.
However, some of the earliest insect fossils already show relative enlargement or reduction of one wing pair, and this is true of most insects today. In anisopterous Odonata (Fig. 2F) ; Orthoptera (Fig. 2C) and winged Phasmida; Blattodea; one family of Isoptera (Mastotermitidae) and Mantodea (Fig. 2M); most Plecoptera, Dermaptera, Trichoptera (Fig. 2A), and Coleoptera; and some Hemiptera and male Strepsiptera (Fig. 2P), the hind wing is significantly broader than the forewing and has become the principal lifting surface. In Ephemeroptera, Psocoptera, some Hemiptera (Fig. 2J) and Neuroptera, many Lepidoptera (Fig. 2L, 2O, and 2R), most Hymenoptera (Fig. 2E, 2H, and 2K), and all Diptera (Fig. 2N), the converse is true; the forewings have greater area and usually greater length than the hind wings. In all but the Odonata, which do not fold their wings, it is necessary for the hind wings to fold up along two or more radiating fold lines to lie beneath the forewings at rest. The forewings themselves are often shorter than the hind wings, and in some cases have become thickened, providing some protection for the abdomen and the more fragile hind wings. When moderately thickened, as in many Orthoptera, Blattodea, Mantodea, and some Homoptera, the forewings are often referred to as tegmina (singular tegmen). When thickened to the extent that their flight function is almost lost or wholly so, as in Coleoptera and Dermaptera, they are known as elytra (singular elytron). The typical forewings of Heteroptera, with the basal part thickened but a membranous tip, are known as hemielytra.
Forewings adapted for protection tend to be less effective in flight and are usually accompanied by disproportionately large, elaborately folding hind wings with expanded anal fans or else by total loss of flight capability. Where the forewings are significantly shorter than the hind, for example, in some Orthoptera, winged Phasmida, Dermaptera, and Coleoptera, two different solutions have been adopted to meet the problem of stowing the hind wings at rest. In Orthoptera, for example, Gryllotalpidae, Tridactylidae, and Tetrigidae, and in Phasmida, the longitudinally folded hind wings project from beneath the tegmina along the back of the abdomen. In Phasmida and Tridactylidae, the narrow remigium of the hind wing itself is thickened and protects the more delicate fan. However, in Dermaptera and Coleoptera and in several genera of Blattodea, the hind wings fold transversely as well as longitudinally, in a variety of complex and mechanically fascinating ways.
Some reductions and modifications are extreme. Among Ephemeroptera, Caenidae and some Baetidae have lost the hind wings entirely and fly with the forewings alone; the same is true of male Coccidae (Homoptera, Fig. 2D). The hind wings of Diptera are modified as gyroscopic sense organs, the halteres, and the forewings of male Strepsiptera (Fig. 2P) are apparently similarly adapted. The hind wings of nemopterid Neuroptera are elongate plumes or filaments. In the nemopterid species that have been investigated, they do not flap, but are spread out behind the insect, apparently acting as physical stabilizers. Similar long, more or less slender hind wings occur in several families of butterflies and moths, but in some species at least they flap in phase with the forewings, sending ripple-like waves along their ” tails. ”
The wings of many small moths, flies, beetles, and wasps are fringed with long hairs, which appear to operate aerodynamically as if they were a continuous membrane. This is taken to extremes in the tiny feathery wings of Thysanoptera (Fig. 2B), ptiliid beetles, nymphomyiid Diptera, and mymarid Hymenoptera, of which most of the lifting surface consists of long hairs, arising from a rodlike rachis. The emarginated wings of orneodid moths (Fig. 2R) consist of a radiating fan of similar feather-like structures, and the pterophorid moths approach this condition, although with fewer plumes.
The forewings of many male Orthoptera in the suborder Ensifera are adapted for sound production and amplification, with distorted venation enclosing large areas of membrane—the “mirrors.” These regions, and their function, are often retained in species whose wings are otherwise reduced. The forewings of some noctuid moths are also adapted for sound production.
Reduction (brachyptery) and loss (aptery) of both wing pairs have happened in many orders. They are particularly frequent in Orthoptera, Blattodea, and Hemiptera, and sometimes occur poly-morphically within a species. The adaptive significance of brachyptery is seldom clear. Short forewings are sometimes retained, apparently as protective structures, as are the elytra of many flightless beetles that lack hind wings.
FUNCTIONS AND DESIGN
The primary function of wings is flight. The wings operate as flexible aerofoils, oscillating as first-order levers over a process of the pleuron, which acts as a fulcrum. They need to be rigid enough to resist unwanted deformation under the forces they receive from the air and the inertial forces from the repeated accelerations and decelerations of flapping, but locally flexible enough to allow the cyclic twisting and bending deformations that are necessary to generate the forces to propel the insect and support its weight in air. Because they have no internal muscles, their shape from instant to instant needs to be controlled partly remotely through the axillae, partly automatically by their own structure: the distribution of rigid and flexible elements, of relief, veins, and flexion lines within the wings themselves.
The Wing Beat
To interpret these aspects of wing morphology, it is necessary to understand the nature of the wing beat. High-speed still and cine photographs of a wide range of insects show that in the downstroke, the wings are usually relatively straight along their length, with a slightly curved section and a slight nose-down twist along the span. In the upstroke, however, the shape of the wings varies greatly, both between species and according to how the insect is at that moment performing.
In nearly all species, the wings twist backward (supinate) to some extent at the bottom of the downstroke and continue supinated throughout the upstroke, twisting forward (pronating) again at the top. The extent to which they can twist varies greatly. The wings of most Diptera, and of zygopterous Odonata, which have slender bases and operate in individual pairs, are able to twist almost onto their backs. The camber becomes reversed, and they are then capable of generating upward, weight-supporting forces on the upstroke as well as the downstroke. This allows them to fly slowly and to hover. At the other extreme, locusts, and probably most other flying Orthoptera, can twist their wings only slightly and get the necessary stroke asymmetry by limited forewing twisting, but mainly by pulling the hind wings fully forward in the downstroke and partly retracting them for the upstroke. Pulling the hind wing forward extends the fan, and the radiating veins within it become compressed and curved like the spokes of an umbrella, giving the wing a cambered section, which is lost as the wing relaxes into the upstroke. Only the downstroke generates significant weight support, and the insects can fly only forward, at a relatively high speed.
Between these two extremes fall many insects whose wings are capable of varying amounts of torsion and which hence display a range of versatility and maneuverability in flight. Broad wings and fore- and hind wing coupling both tend to limit active twisting, but many wings show a degree of passive, elastic torsion along their span, so that the distal part is significantly supinated for the

FIGURE 6 Tibicina hematodes (Cicadidae), early in the upstroke. The forewing is strongly supinated, and ventral bending along a transverse flexion line allows the tip to twist sharply, generating useful lift. Stiffening ridges are faintly visible in the membrane.
upstroke and can generate useful lift. In many insects—Plecoptera, sialid Megaloptera, Mecoptera, many Hemiptera and Hymenoptera, some Trichoptera and Lepidoptera—this is enhanced by a degree of ventral bending along a more or less marked transverse flexion line. Where such a flexion line runs obliquely across the wing, ventral bending serves also to twist the distal part of the wing, enhancing upstroke lift generation and increasing flight versatility (Fig. 6).
The Importance of Wing Camber
A cambered cross section makes a wing rigid to both bending and torsion, but asymmetrically so. A thin, dorsally convex wing is far more resistant to a bending force applied from below than to one from above, as the latter tends to make the edges buckle outward and flatten the section. Furthermore, if the force is applied behind the axis along which the wing naturally twists, the latter twists far more easily if the force is from above than from below. These effects are exploited by many insects to favor ventral bending and supina-tory twisting for the upstroke, whether or not a transverse flexion line is present. There is evidence, moreover, that some insects can actively alter the height of the camber, and hence the wing’s rigidity, by longitudinal bending along the median flexion line, and this may prove to be the latter’s principal function.
Automatic Mechanisms
The mechanisms so far described illustrate that the control of wing shape in flight is to a considerable extent automatic: determined by the local distribution of flexibility and rigidity within the structure, moderated remotely by the controlling muscles at the wing base. Nowhere is this automation better demonstrated than in the wings of dragonflies (Odonata, suborder Anisoptera).
These agile aerial predators have long complex wings (Fig. 2F), extensively corrugated, with several richly branched longitudinal veins and an abundance of crossveins. The principal support is provided by the costa, subcosta, and radius, which are linked together in the basal part of the wing by high-relief, angle-bracket-shaped crossveins (Fig. 3D) into a spar with a V-shaped cross section, rigid to both bending and torsion. About halfway along the wing, the sub-costa terminates at the “nodus,” a high-relief structure consisting of a strong transverse strut and a patch of flexible cuticle that interrupts the costa. Beyond the nodus, the costa and the radius form with the first branch of the radial sector a more shallow spar with an inverted V-shaped section, supporting the distal part of the wing but compliant to supinatory twisting. The crossveins here, and elsewhere in the wing, are slender, allowing considerable deformation, so that the flapping wing twists forward and backward about its leading edge spar, like the sail of a tacking dinghy. The nodus serves to reinforce the wing and to minimize stress concentrations at the transition point between the two, mechanically very different, sections of the leading edge spar. The extreme twisting, essential to its versatile, high-performance flight, is assisted by an unusually flexible trailing edge; but it is essential that this latter should not be allowed to swing up like a flag to flutter in the airflow, which would be aerodynami-cally useless and potentially damaging to the wing. This is automatically prevented by two features: a dense, blood-filled space called the pterostigma, near the end of the wing tip, which is believed to act as a regulating counterweight to the swinging wing, and a curious, three-dimensional formation of veins near the wing base—the triangle and the supratriangle—which automatically levers the trailing edge downward in direct response to the aerodynamic forces that the wing generates. This degree of automation in a propulsive appendage is unique in the animal kingdom and has no obvious parallels in technology.
Wing Folding
All modern insects except Odonata and Ephemeroptera have the ability to fold their wings back over the abdomen, an adaptation that increases their ability to move freely over a substrate and to penetrate small spaces. Simple folding back is achieved mainly by muscular rotation of the third axillary sclerite and folding of the wing base along the basal hinge between the sclerite and the side of the thorax and along the jugal fold line. In hind wings with a large vannus (anal fan), there may be many extra fold lines lying parallel to the radiating veins, so that the wing folds like a pleated fan. The hind wings of Dermaptera, Coleoptera, and the cockroach Diploptera, however, fold up transversely as well as longitudinally, in some cases with extraordinary complexity. Because the wings have no internal muscles, folding and unfolding need to be achieved remotely, by mechanisms operated from the wing base and by the action of other body parts, combined with elasticity within the wing. Hydraulic mechanisms have sometimes been postulated, but recent work suggests that these are unnecessary. The unfolding of beetle wings appears to be achieved by active, scissor-like movements of the bases of the main supporting veins, which set in action a cascade of interconnected, simple opening mechanisms similar to those used in popup topics and three-dimensional greeting cards, assisted in places by elasticity in the cuticle. Refolding the wings involves the reversal of these mechanisms, assisted by movements of the abdomen.
Dermaptera, by contrast, use their abdomens to unfold the hind wings by means of a complex array of similar mechanisms, and refolding is largely elastic.
Secondary Functions
Wings have assumed a range of secondary functions. The sound-producing and -amplifying forewings of many Orthoptera, the protective elytra and tegmina of several orders, and the sensory halteres of Diptera and Strepsiptera have already been mentioned. The wings of Odonata and many Lepidoptera assist in thermoregula-tion. Members of several orders—Orthoptera, Phasmida, Mantodea, Odonata, Diptera, Lepidoptera—use theirs in active signaling: defensive, territorial, and sexual. Wings with warning patterns and colors are found particularly among Coleoptera, Lepidoptera, Orthoptera, and Heteroptera. The use of wings in camouflage is especially widespread in Orthoptera, Mantodea, Heteroptera, and Lepidoptera and may be reflected in their shape as well as color and pattern. Those of leaf mimics may be unusually broad and may bear petiole-like protuberances and emarginations resembling insect damage. It is often hard to determine whether particular wing characters are flight adaptations or have only secondary functions.
MODELS, ANALOGUES, AND APPLICATIONS
Physical, analytical, and numerical models have proved particularly useful in understanding the wings’ unique properties as flexible, smart aerofoils, and the last few years have seen a burst of activity by engineers in investigating and modeling the behavior and aerodynamics of flapping, insect-like aerofoils, and their possible contribution to the development of micro air vehicles: small, semi-autonomous flying robots with a number of potential uses in military and civil contexts.
Unsolved Problems
Many wing characters are still poorly understood. We know far too little about the mechanical properties of the wing materials, and how they vary within wings and between taxa. We are also largely ignorant of the aerodynamic significance of the great variety of wing shapes in, for example, Lepidoptera, and of surface structures like hairs, spines, and scales. While the principles underlying venation pattern and the layout and orientation of flexion lines are broadly clear, the details are often quite obscure. Much work, linking aerodynamics, functional morphology, and structural mechanics, remains to be done.
