Many insects communicate by mechanical signals propagating through a medium as near- or far-field airborne sound, substrate vibrations, underwater sound, and/or water surface vibration. Vibrational signaling through substrate is most commonly used whether counted by species, family or phylogenetic distribution (Fig. 1). Over 195,000 species communicate with substrate-borne signals alone or together with other forms of mechanical signaling and about 150,000 species use vibrational signaling exclusively. Insects emit vibratory signals in connection with aggression, distress, calling, courtship, rivalry, and other specific behaviors. Among many tasks, substrate-borne signaling enables mate location and recognition. This article presents vibratory mechanisms, structures, and signals in relation to behavior.
MECHANISMS OF VIBRATION PRODUCTION
Insects produce vibratory signals by percussion, vibration of the body or its part, tymbal mechanisms, or stridulation. Percussion is common because of the hard exoskeleton, which enables either percussion of two body parts or striking against a substrate. The percussive structures are in most cases relatively simple. Topic lice (Psocoptera) and stoneflies (Plecoptera) tap their abdomens against the substrate, Orthoptera use their legs, and termites and beetles drum with their heads. Tremulatory signals are produced by an insect with legs kept firmly on the substrate and jerky movement of the whole body without touching the substrate. In meadow katydids, larger males produce tremulatory signals with shorter inter-pulse intervals; females prefer sequences with shorter inter-pulse intervals indicating that tremulatory signals encode information on body size. Low-frequency signals can be emitted also by vibration of some body parts. For example, male and female chloropid flies Lipara communicate over distances of more than 2 m on a reed with signals produced by vibration of the abdomen. Lacewings (Chrysopidae) oscillate the abdomen without touching the substrate, and in this way shake the stem or leaf on which they are standing. In such a way, they produce the low-frequency tremulatory component of the broad signal used for communication. Abdominal muscles are involved in song production also in the Hawaiian fly Drosophila sylvestris. Bees produce vibratory signals by thorax vibrations; “begging” signals are transmitted to the substrate through the legs, and “tooting” and “quacking” signals directly by pressing the thorax to the substrate.
Tymbal-like mechanisms are used to produce vibratory signals in leafhoppers, planthoppers, treehoppers, spittle bugs and cicadas. Because there are no special resonant air sacs behind the tymbal, the carrier frequency of emitted sound remains low and suitable

FIGURE 1 The distribution of vibrational signaling among insect orders. Green colour indicates presence; red colour, widespread use of this modality.
for transmission through a substrate. Many insects produce vibratory signals by stridulation as friction of two body parts moved one across another. For example, ants stridulate by means of a file on the dorsal surface of the first abdominal segment and a plectrum on the posterior edge of the metathorax or postpetiole. The substrate-borne component of the audible signal is used for communication. Burrower bugs (Cydnidae) communicate with substrate-borne signals produced with the tergal plate, which functions simultaneously as tymbal, as a file with its laterofrontal surface, or as a plectrum for the alary stridulitrum on the postcubital vein.
Vibratory signals are produced by different insect activities. An insect singing on or close to a substrate produces signals with airborne and substrate-borne components, and many of them use both simultaneously or alternatively. Fruit flies communicate with near-field sound radiated from wings and with substrate-borne signals, which are simultaneously produced by muscles driving the wings. Soil buzz vibrations and sounds produced by solitary bees and wasps are used as cues for localization.
TRANSMISSION OF VIBRATIONS
An insect standing on a substrate may be represented by a model of a mass on six springs. The bodies of treehoppers, spiders, and even fiddler crabs respond to substrate vibration with resonance at lower frequencies and attenuation at higher frequencies. Legs transmit body vibrations to the substrate and are the seat of most sensitive vibrational receptors. Transmission over the legs depends on their stiffness, and the response properties of the body-legs oscillating system vary in different species with the change in posture. Signal amplitude decreases during transmission to the substrate, mainly because the body vibrates during singing also in a horizontal plane.
Green plants represent the most common substrate on which insects feed and mate. They communicate through them by bending waves which are characterized by particle motion in a plane perpendicular to the direction of wave propagation and to the surface with stresses and strains in the longitudinal direction. Insects use dispersive or nondispersive bending waves; the latter are characteristic for communication with high-frequency signals through larger stems. The propagation velocity of bending waves varies little with the structure’s mechanical properties and is proportional to the square root of the structure’s radius and the square root of the frequency. A point on a stem surface moves in an ellipse when viewed relative to a cross-sectional plane; at any frequency, the ellipse’s major axis corresponds to the maximum amplitude of vibration. Vibratory signals travel through green plants with low attenuation, reflect mainly at the root and top of the plant and may travel back and forth several times creating standing wave conditions in rodlike structures such as stems, stalks and side branches. The green host plants act as low pass filter with resonant properties tuned within the range of the fundamental frequency and higher harmonics of signals of most insects communicating through plants. Lowest attenuation with the distance is characteristic for about 100 Hz signals transmitted through green plants. Most insects adapt spectra of their vibratory signals with mechanical properties of transmission medium to optimize the range of substrate-borne communication. Vibratory signal spectra of the southern green stink bug, Nezara viridula, and until now all investigated species of the subfamily Pentatominae have a narrow fundamental and dominant frequency peak between 80 and 160 Hz (Fig. 2). Burrower bugs emit broadband signals composed of the stridulatory and low-frequency components produced by body vibration; the latter unit contains most of the emitted energy. Carpenter ants, Camponotus herculeanum, live in tree trunks from which the soft spring wood has been eaten out, leaving thin, lignified lamellae. The alarm signals, produced by drumming the head and gaster against the substrate, are particularly suited for transmission through the nest but not through to the outside. “Begging signals,” emitted by bees following a dancer, are used to solicit food samples. The 1-pm peak amplitude of the signals lies slightly above the threshold for the bee ” freezing response,” and the signal dominant frequency around 320 Hz lies in the range of the best signal-to-noise ratio of the bee comb.
Investigations of other transmission media for insect vibratory signals are rare. The European pit-building antlions, Euroleon nostras, uses sand to detect prey. Sand particle size and the frequency of prey signals influence their propagation properties: damping is inversely proportional to the size of sand particles and is positively correlated with the frequency. The reaction distance is shorter compared with green plants and ranges from a few centimeters in the finest sand to about 12 cm in coarser one.
RECEPTOR MECHANISMS
The most sensitive and specialized receptor for substrate vibrations, the subgenual organ, is derived evolutionarily from chordo-tonal organs in the body and appendage joints. It has been found in all Pterygote insects, except Coleoptera and Diptera. The organ lies in insects in all the six legs close to the main tracheal trunk and almost completely occludes the dorsal blood sinus of the proximal tibia. The organ is supported proximally by the nerves and distally by accessory cells that form a ligament attached most often to the cuticle of the leg. The structural unit of the organ is the scolopidium, each of which is composed of one or more bipolar sensory cell(s) and two accessory cells, the scolopale and the cap (attachment) cell. The hemolymph dampens the movement of freely suspended neuron side of the scolopale, while no such dampening occurs at the side anchored to the cuticle.
In Orthoptera, the subgenual organ is accompanied by other scolopal organs of which the crista acoustica, supported by sound-transmitting structures, represents a very sensitive receptor for airborne sound. In the American cockroach, Periplaneta americana, the subgenual and the neighboring Nebenorgan (Fig. 3) are most sensitive to substrate vibrations. The distal organ, which surrounds the hemo-lymph, responds to variations in hemolymph pressure. Scolopidia of the subgenual organ are organized in a fan-shaped manner and converge radially toward a single insertion point so that vibrations of the substrate cause tension changes. The organ is most sensitive in the frequency range between 1000 and 5000 Hz, with threshold amplitudes around 0.1 nm. Scolopidia of the Nebenorgan are stretched perpendicular to the long axis of the tibia so that the organ reacts to tension changes within the scolopale caused by vibration of the cuticular walls.
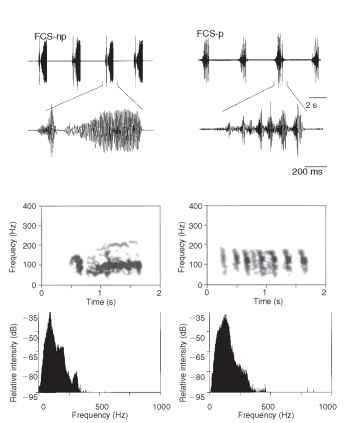
FIGURE 2 Oscillograms (top), sonograms (middle), and power spectra (bottom) of the nonpulsed (left) and of the pulsed (right) type of N. viridula female calling song.
Subgenual organs of different morphology have been described in different insect groups. In the southern green stinkbug, the sub-genual organ is proximally attached to the epithelium of the tibial wall, whereas the two scolopidia with the cilia and the flat and thin ligament are stretched out in the hemolymph of the blood channel. The subgenual organ in the legs of the lacewing Chrysoperla carnea (Fig. 4) is composed of only three scolopidia. The cell bodies of the three cap cells form a lenslike part of the organ, the velum, which dis-tally divides the blood channel into two separate parts. Scolopidia are attached to the middle of the velum and extend to the dorsal leg wall. The hollow cone-shaped bee subgenual organ with approximately 40 scolopidia is connected at two points to the cuticle and at two points to the membrane bag surrounding the organ and the membrane lining the tracheal wall. The organ oscillates with the hemolymph, and the sensory cells respond to displacements of the organ relative to the leg.
The complex tibial organ of bushcrickets is composed of the sub-genual organ, intermediate organ and crista acoustica. The latter and the distal part of the intermediate organ function as the most sensitive auditory organ only in forelegs; the same organs in mid- and hind legs respond to vibratory stimuli like in forelegs, but unspecifically to airborne sound. The functions of the middle and distal parts of crista acoustica in mid- and hind legs have not been described.
Mechanoreceptors such as campaniform sensilla, joint chordotonal organs, and Johnston’s organ respond to substrate vibrations with lower sensitivity, preferentially in the frequency range below 100 Hz.
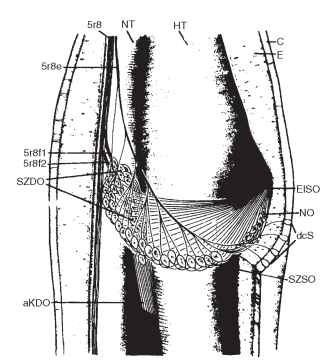
FIGURE 3 Subgenual organs of the cockroach P. americana. The left tibia is opened to show the spatial arrangement of the scolopidial organs. aKDO, accessory cap cells of the distal organ; C, cuticle; dcS, distal campaniform sensilla; E, epidermis; EfSO, terminal fila of the subgenual organ; HT, main trachea; NO, Nebenorgan; NT, small trachea; SZDO, sensory cells of the distal organ; SZSO, sensory cells of the subgenual organ; 5r8e, nerve innervating the subgenual organs and the campaniform sensilla.
NEURONAL PROCESSING
Compared with processing of auditory information, less data are available about morphology and function of higher order neurons responding to stimulation of vibrational receptors. In the stinkbug, N. viridula. two receptor neurons of the subgenual organ in each leg together with low-frequency receptor neurons of the joint chor-dotonal organs and campaniform sensilla give rise to more than 20 different interneurons at different levels of the ventral cord. Most of them are tuned to frequencies below 400 Hz, which is the characteristic frequency range of pentatomine vibratory signals. Some of these interneurons discriminate between signals coming from different directions by analyzing the time pattern of different legs’ stimulation. Similar morphology of several interneurons in Orthoptera groups with or without crista acoustica indicates that hearing developed in insects through the evolution of the sense of vibration. In Orthopteran families Acrididae and Tettigoniidae, the auditory and vibratory information received from the tympanal and vibrational receptor organs converges to the same interneurons which are classified according to relative sensitivity to each modality as predominantly sound, vibration or sound, and vibration neurons. Simultaneous processing of the auditory and vibratory information within bimodal interneurons in many circumstances optimizes signal resolution.

FIGURE 4 Three-dimensional reconstruction of the subgenual organ of the left middle tibia of the lacewing C. carnea. BC, blood channel; C, cap; CC, cap cell; CU, cuticle; S1, S2, S3, three scol-opidia; SC, scolopale cell; SE, sensory cell; TR, trachea; V, velum.
VIBRATORY SIGNALS AND INSECT BEHAVIOR
Insects emit vibratory signals in connection with aggression, distress, calling, courtship, rivalry, and many other behaviors. In dense vegetation wind, local air currents, distance and plant architecture prevent reliable communication with signals other than those transmitted through a plant. Social insects such as bees, ants, and termites live in nests where the possibility of communication by signals of other modalities (other than airborne chemicals) is limited. Insects, whose small body size does not allow efficient low-frequency sound radiation, can use vibrational communication without attracting predators or parasites as they would by singing. Many insects use the vibratory component of the emitted airborne signals to improve signal discrimination and recognition. Emission of vibratory signals can help herbivores to avoid predation. When a predator approaches, for example, treehopper nymphs, the nearest one produces a brief vibra-tional signal which triggers emission by others in the group as a chain reaction. This longer and louder group signal attracts the mother to move into the offspring aggregation to defend her young. Vibratory signals of Riodinidae butterfly larvae in concert with chemical signals attract mutualistic ants which provide protection from predators.
Vibrational communication through plants is disturbed by environmental factors such as wind and raindrops. For example, wind induces low-frequency vibrations that are accompanied in apple leaves with broadband vibrations of spectra up to 25 kHz. Raindrops falling on banana leaves produce vibrations up to 1000 Hz; those on apple leaves cause vibrations composed of an irregular high-frequency and a regular low-frequency phase. Signal-to-noise ratio is enhanced by the highly ordered temporal structure of songs with narrower spectra with dominant frequencies, usually above the low-frequency noise level, as well as by band-pass filtering properties of the subgenual organ.
The time pattern of a vibration pulse series usually carries more information about signal specificity than its spectral structure. Generally, a rapid signal divergence occurs in sympatric taxa, and song similarity is expected only in allopatric or allochronic species. In Chrysoperla, tremulation songs represent the best cue for species identification within the carnea group. Within the same region, the songs of different cryptic species differ structurally and functionally more than in pairs of species from North America and Eurasia. The vibratory song repertoire of sympatric Californian species N. viridula and Acrosternum hilare differ, and interspecific mating has not been observed. In Japan, however, the sympatric species N. viridula and N. antennata mate although their songs differ.
Vibratory signals are used by insects also as cues for mate, prey, or enemy localization. In N. viridula, male directional movement on a plant results from female calling song signals. Leaf-cutting ant, Atta cephalotes, workers stridulate when they cut leaves, and nearby ants respond by orienting toward the source of the vibrations and join in the leaf cutting. The response of the heteropteran predator Podisus maculiventris to vibrational signals produced by a common prey species demonstrates that these predators are capable of using substrate-borne vibrations as cues for prey location. The male katydid, Conocephalus nigropleurum, shakes its body to produce vibratory signals that attract females during courtship and mating.
Central nervous resolution of stimulation time differences and amplitude gradients, which occur when vibration travels through a substrate from one leg to another, is the mechanism that enables directionality in larger insects. For example, the response of the body of the treehopper Umbonia crassicornis relative to the substrate reveals resonance at lower frequencies and attenuation at higher frequencies. The transfer functions measured on the body differ substantially depending on whether the stimulus originates in front or behind, indicating that directional information is available in the mechanical response of the body to substrate vibration. Comparison of signal amplitudes between receptors of the front and back legs might enable vibration localization in smaller insects.
