Salivary glands are glands associated with the mouth or oral cavity and produce secretions (saliva) that are mixed with the food during feeding and are ingested along with the food. Among insects, there are four pairs of glands associated with the mouth or oral cavity, although all four generally are not present in the same insect. Each of these is associated with and is named after its associated mouthpart: the mandibular, maxillary, hypopharyngeal, and labial glands. The presence or absence of each of these glands varies among insect species, and in holometabolous insects, a given gland may be present in only one life stage. For example, in Lepidoptera, mandibular glands occur in the larval stage, but not in the adult. The four glands serve a variety of functions, including some non-salivary functions, and the same gland may serve different functions in different species or even in different life stages of the same species. Within a given species, one or more of these four pairs of glands usually function as salivary glands.
GLANDS ASSOCIATED WITH THE MOUTH
Mandibular glands, found in many insects, function as the main salivary glands in Lepidoptera larvae. A common function of mandib-ular glands in social Hymenoptera is to produce pheromones such as alarm pheromones in ants and honey bees, and the queen substance in queen honey bees. Function can vary with age. For example, the mandibular glands of older honey bee workers (which perform mostly foraging tasks) produce an alarm pheromone, whereas the mandibu-lar glands of young workers (which perform mostly nursing tasks) produce secretions, called royal jelly, that are fed to larvae in differential quantities to control whether a given larva will develop into a queen or worker. Mandibular glands of stingless bees serve in defense and produce a burning sensation when ejected onto the victim.
Maxillary glands occur in Protura, Collembola, some Heteroptera, and some larval Neuroptera and Hymenoptera. They are believed to provide secretions to lubricate the mouthparts, and thus serve one of the salivary functions.
Hypopharyngeal glands occur in the Hymenoptera, and in honey bee workers they produce secretions that are fed to the larvae (a substance different from royal jelly). Hypopharyngeal glands are vestigial in honey bee queens and absent in males. The hypopharyngeal glands of honey bee workers also produce invertase, a common salivary enzyme that hydrolyzes sucrose, and another enzyme that oxidizes glucose to an acid, which is believed to serve as a preservative in honey.
Labial glands occur in the great majority of insect orders (an important exception is the Coleoptera), and most commonly function as salivary glands, but in the larval stage of some groups of silk-producing insects, such as Lepidoptera, Trichoptera, and Hymenoptera, labial glands are the silk-producing organs either throughout the larval stage or at its end, just before pupation. In the Psocoptera, adults have two pairs of labial glands, one pair functioning as silk glands and the other pair functioning as salivary glands. In the primitive orders Collembola and Thysanura, which lack Malpighian tubules (the usual excretory organs of insects), the labial glands function as excretory organs.
STRUCTURE AND FUNCTION OF SALIVARY GLANDS
The salivary glands of most insects are labial glands, which are the focus of this section. Labial salivary glands have been examined in detail in relatively few insect species, and there is great variation among the species examined (Fig. 1). This is not surprising, considering the great variation in mode of feeding (e.g., chewing, piercing-sucking, non-piercing-sucking, sponging, etc.) and types of food consumed by different insect species.
General Description
Several aspects of structure and function are common to most or all variations of insect labial salivary glands. The glands occur in pairs, and the ducts from each gland usually join to form a single common duct that opens to the oral cavity at a single orifice (Fig. 1). Even though the glands originate in the labial segment, the orifice usually occurs just behind or on the hypopharynx, and the glands often extend back into the thorax and even as far back as the abdomen (e.g., Fig. 1A and 1B). The glands are suspended in the hemocoel and are constantly bathed in hemolymph. The glands generally have at least two regions: a secretory region and a reabsorptive region. Generally, the lumen of the salivary duct is lined with cuticle, at least at the end closest to its opening.
The secretory region produces the primary saliva. The major component of saliva is water. Water is transported from the hemo-lymph across cells of the salivary gland and into the lumen of the gland. Movement of water from the blood to the gland lumen is accomplished by active transport of potassium or sodium ions from the hemolymph to the lumen, causing water to move from the hemo-lymph to the lumen down an osmotic gradient. Cells responsible for water transport generally have deep infoldings of the cell membrane and/or dense microvilli on the side of the cell adjacent to the lumen of the gland. This serves to greatly increase the cell’s luminal surface area, and also serves to enclose very narrow extracellular spaces into which ions are pumped. The enclosed nature of the spaces helps contain the ions to keep their concentration high, thus facilitating the osmotic movement of water from the cell into the space. The infold-ings and microvilli usually are associated with abundant mitochondria to provide the energy for the ion pumps. The secretory region of the gland also synthesizes proteins, such as salivary enzymes and other organic components of the saliva. Cells responsible for secretion of these components generally possess extensive endoplasmic reticulum, Golgi bodies, and secretory granules that synthesize and transport (intracellularly) the secretions. There may be one or several different types of cell in the secretory region. It should be noted that salivary components are not necessarily produced by the salivary
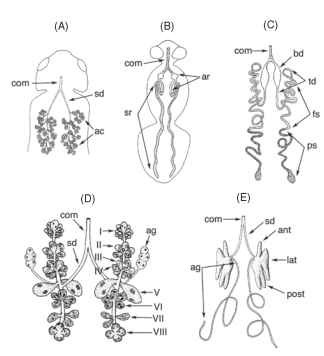
FIGURE 1 Salivary glands of representative insects. (A) The locust, Locusta. (B) Adult blowfly, Calliphora. (C) The tobacco hornworm moth M. sexta. (D) The beet leafhopper, C. tenellus, showing cell types I through VIII. (E) The large milkweed bug, O. fasciatus. Abbreviations: ac, acini; ag, accessory gland; ant, anterior lobe; ar, absorptive region; bd, bulbous duct region; com, common salivary duct; fs, fluid secretion region; lat, lateral lobe; post, posterior lobe; ps, protein secretion region; sd, salivary duct; sr, secretory region; td, thin duct region.
glands themselves, but may be produced elsewhere in the body and transported to the salivary glands via the hemolymph.
The reabsorptive region of the salivary gland reabsorbs potassium or sodium ions from the saliva and transports them back into the hemolymph. As a result, potassium and sodium ions are conserved, and the saliva is usually hypotonic to the hemolymph. Reabsorptive cells often have infoldings, especially on their basal side (hemolymph side), to increase surface area. These infoldings, however, tend not to be tightly enclosed (unlike the lumen side of water-secreting cells in the secretory region), to facilitate movement of secreted ions into the hemolymph and away from the cells, thus reducing the osmotic gradient, which would cause the cells to lose water. Reabsorptive cells also have abundant mitochondria to power the active transport of ions from saliva to hemolymph.
Acinuous Salivary Glands
The salivary glands of many insects are composed of clusters of acini, or saclike glandular structures (Fig. 1A) comprising at least two types of cell. Each acinus empties into a cuticle-lined duct, and the ducts of different acini fuse, eventually forming a common duct that leads to an opening just behind or on the hypopharynx. The anatomy of acinuous salivary glands varies greatly among different insects. The acinuous glands of locusts and cockroaches have been particularly well studied and are described next.
LOCUSTS AND COCKROACHES
The gross anatomy of
locust and cockroach salivary glands is illustrated in Fig. 1A. The acini are the secretory region of the salivary gland and consist of peripheral cells and central cells. The peripheral cells have deep, microvilli-lined invaginations that are continuous with extracellular canaliculi that open into the gland’s duct. The peripheral cells transport water from the hemolymph to the canaliculi, and from there, the water empties into the duct. Additionally, in locusts, the peripheral cells seem to synthesize and secrete other salivary components. The central cells of both locusts and cockroaches synthesize salivary enzymes and other salivary components, and secrete them into the salivary duct. The walls of the salivary ducts of locusts and cockroaches are one cell thick, and the lumen is lined with a thin cuticle. The salivary duct contains the reabsorptive region of the gland, and in some cockroaches, a small part of the duct adjacent to the acinus is secretory.
Tubular Salivary Glands
Some groups of insects, such as Lepidoptera, Diptera, and Siphonaptera, possess tubular salivary glands. Generally, the walls of tubular salivary glands are one cell thick and the lumen is lined with thin cuticle. Tubular salivary glands are divided into several regions along the length of the gland. The number of regions and anatomical details of the regions vary among insect groups. Two examples of tubular salivary glands are described.
BLOWFLY ADULTS
There is a pair of tubular salivary glands, each gland with three regions (Fig. 1B) . The apical region is the longest and is the secretory region; the shorter middle region is the reabsorptive region; and the short common duct at the proximal end opens to the exterior of the body on the hypopharynx.
The blowfly’s secretory region comprises a long distal section located mostly in the abdomen and a shorter proximal section located in the thorax. Cells in the distal section serve a dual function: they move water from the hemolymph into the lumen of the gland, and they synthesize and secrete salivary enzymes and other salivary components. The cell surface adjacent to the lumen of the gland’s central duct encloses extensive canaliculi into which the cells secrete their products. Secretions then move from the canaliculi to the gland’s duct. Cells in the proximal section are generally similar in appearance to those in the distal section, but they do not contain secretory granules. Thus, they do not seem to secrete enzymes and probably secrete only water and ions. The reabsorptive region of the blowfly’s salivary glands consists of a single cell type that is believed to be responsible for reabsorbing ions from the saliva back to the hemolymph. Finally, the common duct of the blowfly’s salivary glands comprises highly flattened cells that seem to play no role in the secretion or reabsorption of any salivary components. This region of the salivary gland is very short and opens on the hypopharynx.
SPHINGID MOTH
In the tobacco hornworm, Manduca sexta, adults have a pair of tubular salivary glands that are divided into four regions (Fig. 1C). In the apical region, proteinaceous material is synthesized in the extensive rough endoplasmic reticulum and Golgi bodies, and is stored in large vacuoles before eventual release into the lumen of the gland. The second region transports water from the hemolymph to the lumen. Cells in this region have the characteristic structure of water-secreting cells to accommodate what is believed to be the primary function of this region; but in addition, the presence of rough endoplasmic reticulum and Golgi bodies suggests that these cells secrete more than just water. The third and fourth regions, called the thin duct and bulbous duct, both seem to have a reabsorptive function, moving ions back from the saliva and into the hemolymph. Cells in these two regions differ in the structure of their surface adjacent to the lumen, but the reason for the difference is unknown. After the fourth region, the right and left glands fuse forming the common duct. Cells of the common duct are unspecial-ized and probably play no role in saliva production.
Salivary Glands of Hemiptera
The most complex insect salivary glands that have been studied occur in the Hemiptera. This complexity is undoubtedly related to the piercing-sucking mode of feeding in this taxon, where saliva is injected into the food substrate via a specialized salivary canal in the elongate maxillary stylets. In this mode of feeding, solid substrates must be pierced and then the food, which is often initially solid, must be liquefied before ingestion through the maxillary food canal. These processes depend greatly on a multitude of salivary components that serve different functions. Consequently, there are usually many distinct types of secretory cells in the same gland, each producing different salivary components. Furthermore, many phytophagous hemipterans produce two distinct types of saliva at different times in the feeding process: sheath saliva and watery saliva. Sheath saliva consists mostly of lipoprotein and is secreted incrementally as the stylets advance through the plant tissue. It gels shortly after secretion and forms a continuous solid sheath around the stylets. As a consequence, only the stylet tips come in direct contact with the plant tissue; the shaft of the stylet bundle is encased by the sheath. Watery saliva, as the name implies, is dilute and does not gel. It contains mostly water and various enzymes.
In general, salivary glands of the Hemiptera are divided into two main parts, the principal gland and the accessory gland. The principal gland is often subdivided into two or more lobes. The principal and accessory glands are served by their respective ducts, and the ducts from each lobe fuse to form a lateral salivary duct. The lateral ducts from each side fuse to form a median duct that leads to the salivary pump (described later in this article). There is considerable variation in the salivary glands among the Hemiptera, even within the same family. Two examples are described.
BEET LEAFHOPPER
The principal salivary glands of the beet leafhopper, Circulifer tenellus. are divided into an anterior and posterior lobe, each served by its own duct (Fig. 1D ) . The ducts from each lobe fuse to form the lateral salivary duct. The accessory gland is not subdivided, and its duct joins the others near the point where they fuse to form the lateral duct. The principal glands have eight different cell types that are arranged in rings around the duct of each lobe, five in the anterior lobe and three in the posterior lobe. All eight cell types are secretory, possessing abundant endoplasmic reticulum and/or secretory granules, and all have intracellular canal-iculi that come in contact with the salivary duct. Details of the cells’ fine structure and the staining properties of the secretory granules differ sufficiently to indicate that each cell type produces different components of the saliva. Accessory gland cells have some features typical of water-secreting cells but also have abundant endoplasmic reticulum, Golgi bodies, and secretory granules, suggesting that they secrete water and other salivary components.
MILKWEED BUG
As in most hemipterans in the suborder Prosorrhyncha, the principal salivary glands of the large milkweed bug, Oncopeltus fasciatus, (Fig. 1E) are divided into discrete lobes that are much more compact than the lobes just described for the beet leafhopper. The lobes comprise a mass of cells with a distinct glandular lumen. The accessory glands and the three lobes of the principal glands each secrete their own salivary components. The anterior and lateral lobes secrete two different components that mix to form the salivary stylet sheath (described earlier). The lateral lobe secretes the bulk of the sheath saliva protein, including most of the components that form the hydrogen bonds that solidify the salivary sheath, while the anterior lobe secretes the components that form most of the disulfide bonds. The posterior lobe produces salivary digestive enzymes, such as amylase and esterase. The accessory gland supplies the bulk of the water in the saliva, as well as polyphe-noloxidase and possibly mucoid substances.
CONTROL OF SECRETION, INNERVATION
Secretion of saliva generally is stimulated either by direct inner-vation or by neurohormonal factors that are released into the blood by secretory neurons. Innervation of the salivary glands varies considerably among different insects. Innervation can come from the subesophageal ganglion, thoracic ganglia, the stomatogastric nervous system, the median-transverse nervous system, or a combination of these.
Two neurotransmitters, serotonin and dopamine, are commonly found in neurons innervating the salivary glands, and each may stimulate different aspects of salivation. For example, in the American cockroach, serotonin induces secretion of proteinaceous saliva, whereas dopamine induces secretion of nonproteinaceous saliva. Other neurotransmitters occur in neurons innervating salivary glands, but their roles are not understood. The diversity of neuro-transmitters suggests that control of salivation is a complex process.
The salivary glands of some insects such as blowflies lack direct innervation, and salivation is induced by one or more neurohormo-nal factors released into the blood. One of these factors seems to be serotonin.
STRUCTURES ASSOCIATED WITH SALIVARY GLANDS
Salivary Reservoirs
Some insects, such as cockroaches, have a pair of distensible salivary reservoirs for storage of saliva. In cockroaches, each reservoir has its own duct, which joins with the duct of its associated salivary gland. The combined gland/reservoir ducts from each side fuse to form the common salivary duct leading to an opening on the hypopharynx. A valve near the orifice of the common duct on the hypopharynx opens and closes as the hypopharynx is raised and lowered during feeding, thus controlling release of saliva. When the insect is not feeding, the hypopharynx is in a lowered position, closing the valve, and saliva produced by the salivary glands then backs up into the reservoirs where it is stored.
Salivary Pumps
Many insects with piercing-sucking mouthparts inject saliva into their food for various purposes. Often a pumping mechanism is used to inject the saliva through elongate hypodermic needle-like mouth-parts. In many Hemiptera, the pump is a hollow chamber near the hypopharynx and is referred to as a salivary pump or salivary syringe.
FUNCTIONS OF SALIVA
General
Perhaps, the most fundamental and ubiquitous function of saliva in insects is lubrication of the mouthparts and lubrication of the food bolus to assist its transport through the foregut. Lubrication can be achieved primarily by water, the most abundant constituent in saliva. Water in the saliva also can dissolve components in the food, such as sugars, which then become detectable by chemoreceptors on the mouthparts. Thus, saliva also can aid in food recognition.
The most common class of organic constituents of saliva consists of digestive enzymes, such as amylase, invertase, various proteases, and lipases. In many insects with chewing mouthparts, salivary enzymes are mixed with the food during chewing and swallowing, and initiate digestion. However, the midgut usually is the main site of production and secretion of digestive enzymes, and salivary enzymes in these insects generally play only a secondary role in digestion. In other insects, salivary digestive enzymes provide the main digestive function. This is especially common in piercing-sucking insects, such as many Heteroptera, in which digestive enzymes are injected into the food. The enzymes then break down and liquefy the food, and the digested, liquefied food is sucked up through the mouthparts.
Predators
Predaceous insects that have piercing-sucking mouthparts often capture and eat prey that are as big or even bigger than themselves. Large prey size is not nearly as common in predators with chewing mouth-parts. This is because many predators with piercing-sucking mouthparts use the mouthparts to inject a salivary toxin into the prey, which enables them to subdue prey without having to be large and strong enough to physically overpower them. Thus, in these insects, saliva assists prey capture and gives the insects a potentially larger range of prey than their chewing mouthpart counterparts. Venoms in predaceous piercing-sucking insects often are accompanied by salivary hyaluronidase, which breaks down hyaluronic acid, an important “intercellular cement” in insects. Hyaluronidase is believed to serve as a spreading agent for toxins (as well as for digestive enzymes), assisting their penetration between cells by breaking down the intercellular cement.
Blood Feeders
Salivary components of blood-feeding insects serve several functions. These have been best studied in mosquitoes, other biting flies, and kissing bugs. Blood feeding is the most dangerous time in the lives of these insects, and survival is greatly enhanced by the ability to complete the task quickly and escape before being detected by the host. Salivary enzymes help to shorten the time it takes to acquire a blood meal. Blood vessels occupy only a small volume of skin tissue, and thus locating blood with the mouthparts can take considerable time. During probing, many blood feeders damage capillaries or tiny blood vessels by random movement of the stylets, and small subcutaneous pools of blood (hematomas) form in the vicinity of the damaged vessels. This increases the volume of the blood in the skin, and thus increases the probability that the stylets will locate a blood source, reducing the time required to locate blood. Successful formation of hematomas is greatly facilitated by factors that inhibit blood clotting, and consequently, the most common salivary components in these insects are factors that inhibit clotting. Clotting comprises two general processes, platelet aggregation and coagulation. In the small vessels used by most blood-feeding insects, platelet aggregation is the more important of the two, being capable of plugging a damaged vessel in a matter of seconds. One of the compounds that initiates platelet aggregation is ADP, and the saliva of many different blood-feeding insects contains apyrase, which is an enzyme that breaks down ADP. The importance of apyrase is demonstrated in mosquitoes, where the time required to complete a blood meal is directly dependent on the amount of apyrase injected by the mosquito. In addition to salivary components that interfere with platelet aggregation, some blood-feeding insects have salivary components such as antithrombins that inhibit coagulation. Also, substances that inhibit vasoconstriction occur in some blood-feeding insects, thus inhibiting the host’s attempt to restrict the flow of blood to the feeding site.
Anticlotting also serves another function: it prevents blood from clotting in the food canal. Clotting would clog the food canal and lead to starvation and death. Finally, antihistamines in the saliva of some blood feeders may act as anti-inflammatory agents and reduce the probability that the feeding insect will be detected by the host.
Herbivorous Hemipterans
Many or most phytophagous hemipterans produce two kinds of saliva, sheath saliva and watery saliva, which were described briefly earlier. Sheath saliva forms a continuous solid sheath around the stylets, and several functions for it have been proposed. One likely function is to reduce friction between the stylets and plant tissue, facilitating advancement and withdrawal of the stylets. Another function may be to shield the moving stylets from plant cells, and thus avoid triggering a defensive response by the plant that could include hypersensitive reactions or release of plant defensive chemicals. This may be especially important in sternorrhynchan hemipterans like aphids and whiteflies, which feed primarily on sap from phloem sieve elements that lie deep in plant tissue. These insects carefully weave their stylets between and around plant cells from the plant surface to the sieve elements, and successful extraction of sap from the sieve elements may be dependent on avoiding the triggering of plant defensive responses during penetration to the sieve elements. In addition to mechanically shielding the stylets from plant cells to avoid plant defenses, salivary sheaths contain the enzyme polyphenoloxidase, which has been proposed to serve the function of oxidizing plant defensive chemicals and converting them to more harmless forms.
Watery saliva contains assorted enzymes and other components that vary among species. Some, like proteases and amylases, serve a digestive function, breaking down insoluble plant constituents into soluble forms that can be ingested through the stylet food canal. Others, like pectinases, break down pectin, which is the “intercellular cement” in plants, and loosen the adhesion between adjacent cells. For hemipterans like aphids and whiteflies, whose stylets penetrate between cells until they reach their actual ingestion site (phloem sieve elements), pectinase may facilitate the penetration of stylets between cells by loosening the intercellular cement. After penetrating a phloem sieve element, aphids and whiteflies inject saliva into it. This recently has been demonstrated to prevent sieve elements from plugging up, a plant defense against sap loss, analogous to blood clotting in animals. The water component of watery saliva also can have a critical role other than simply serving as a carrier for enzymes. Many phytophagous hemipterans (especially phytophagous Heteroptera) feed by a method known as “lacerate and flush.” In this feeding method, the stylets are inserted into the plant, and a pocket of cells beneath the surface is liquefied by the combined action of digestive enzymes in the saliva and mechanical laceration by repeated thrusts of the stylets. Once the pocket of cells has been liquefied, the nutrient-rich liquid is flushed out of the pocket by copious secretion of watery saliva and sucked up through the stylet food canal.
Gall Formers
Plant galls are produced by several groups of phytophagous insects that occur especially, but not exclusively, in the dipteran family Cecidomyiidae and the hymenopteran family Cynipidae, as well as many species of sternorrhynchan Hemiptera and some Thysanoptera. The abnormal tissue and cell growth characteristic of plant galls is caused by secretions from the insect, usually salivary secretions, that mimic plant growth hormones or serve as molecular signals that redirect plant cell growth from its normal course to an abnormal form that serves the needs of the gall maker.
Construction of Shelters and Webs
Silk is produced by many insects for a variety of functions such as construction of pupal cocoons in many Lepidoptera, Hymenoptera, and Siphonaptera, and construction of larval retreats or food-gathering nets in most Trichoptera larvae and some chironomid larvae. Many Psocoptera use silk to construct sheetlike shelters under which they aggregate and also use silk to attach their eggs to the substrate. Weaver ants use silk to tie together leaves to construct their arboreal nests, but interestingly, only larvae produce silk; so to weave leaves together with silk, the workers hold the larvae in their jaws and use them as silk dispensers. Not all insects that produce silk do so with their labial glands, but all the examples just cited do, representing some of the diverse uses of these specialized salivary secretions.
Mucoid secretions are produced by the salivary glands of several groups of Diptera. Some Diptera, such as Drosophila, produce salivary mucopolysaccharides that glue the puparium to the substrate. In some sciarids and fungus gnats (Mycetophilidae), mucoid secretions are used as a “slime trail” to facilitate larval locomotion, much like terrestrial snails. Larvae of some predaceous fungus gnats use these sticky mucoid salivary secretions to capture prey. The most fascinating of these are the New Zealand glow worms, which construct a silken retreat from which they dangle silk threads that are covered with sticky mucoproteins to trap prey. The larvae reside in their silken retreats, and at night, they produce light by bioluminescence to attract nocturnal flying insects to their traplines.
Trophallaxis
Trophallaxis is the exchange of food between two individuals. The food exchanged may be salivary secretions or regurgitated gut contents. Larvae of many ants and wasps are dependent on adults to feed them. In exchange for being given food by the adult, the larvae of many species secrete a salivary fluid that is greedily consumed by the adult that provided the food. This stimulus for adults to give up food to a larva may have played a role in the evolution of eusociality in the Hymenoptera, in which adult females readily feed larvae that are not even their own offspring.
Others
Larval warble flies bore their way through the subcutaneous tissues of their mammalian hosts. To facilitate movement through the host’s tissues, they secrete a salivary collagenase, which breaks down collagen, a main component of connective tissue.
The saliva of some moths contains an enzyme called cocoonase that weakens the silk cocoon. It is produced by the newly eclosed moth to assist its escape from the pupal cocoon.
![tmp51-19_thumb[2] tmp51-19_thumb[2]](http://lh6.ggpht.com/_X6JnoL0U4BY/S8Hv4vVa6OI/AAAAAAAAYpY/BxFsJ5wfKX8/tmp5119_thumb2_thumb.jpg?imgmax=800)
