Specialized Terms
Plasmodium Genus (Order Coccidiida, Family Plasmo-diidae) contains more than 100 species of blood parasites of vertebrates, of which Plasmodium malariae, P. vivax, P. falciparum, and P. ovale cause malaria disease in humans.
Anopheles A genus of mosquitoes (Order Diptera, Family Culicidae) that contains 422 species, of which 40 may be involved in the transmission of human Plasmodium.
sporozoites Infective stage of the malaria parasite for the human host, inoculated with saliva by Anopheles females during blood feeding gametocytes Infective haploid blood stage of the malaria parasite acquired by mosquitoes during blood feeding. Sexual union of micro- and macrogametes occurs in the midgut of the definitive mosquito host.
vectorial capacity The epidemiological efficiency of Anopheles host species in transmitting malaria parasites, expressed as new infections per infection per day, based on mathematical relationships among mosquito daily survival, blood meal host selection and feeding frequency, and susceptibility to parasite infection.
malaria paroxysm A clinical attack of malaria in human host, associated with the liberation of parasites from the red blood cells, featuring cold (shivering, lasting <1h), hot (fever as high as 41°C, 2—6h), and sweating (fever breaks, temperature drops rapidly to or below normal) stages.
Malaria is a pyrogenic (fever-producing) disease caused by infection with one of four species of parasitic protozoa in the genus Plasmodium and is the most important arthropod-transmitted pathogen in the world today, in terms of numbers of cases, deaths, and economic burden. Acquired from the bite of an infective Anopheles mosquito or from infected blood products, malarial parasites continue to suppress development in Africa and parts of Asia, and are emerging as a critical health issue in tropical Central and South America. Anthropogenic environmental changes associated with subsistence agriculture have enhanced epidemiological receptivity and endemicity in the New World. Expanding and rapid global commerce and travel provide an effective conduit for malaria parasites to be re-introduced into currently malaria-free areas.
HISTORY AND DISCOVERY
There is little doubt that there has been a long evolutionary association between humans and malaria. The ascent of the human species and their dispersal from the African center of origin into Europe and Asia most likely was accompanied by host-specific and co-evolved species of plasmodia. Vivax malaria possibly accompanied early Asian voyagers to the New World across the Pacific Ocean, whereas falciparum malaria probably was introduced into the New World from Africa with the post-Columbus slave trade.
Malarial disease has impacted human health throughout recorded history. References to seasonal intermittent fevers abound in the earliest Assyrian, Chinese, and Indian religious and medical writings; however, it was not until the 5th century B.C. that Hippocrates related the distribution of cases to specific seasons and residence near marshes. Malaria has altered the course of human history by afflicting political, scientific, and religious leaders as well as decimating invading armies. The Romans associated marshes with intermittent fevers and attempted to reduce their occurrence through swamp drainage. The term “malaria” was derived from the Italian “maV aria” (bad air), drawing from the association between foul-smelling marsh gases and the occurrence of this disease. In the 1600s, powders from the bark of the Peruvian “Quina-quina” tree (now known as quinine) were discovered in South America and shown to be therapeutic against certain seasonal fevers. Shortages of these natural medicinal powders and the resulting impact of malaria on military campaigns during World War I stimulated research to develop antimalarial drugs and resulted in the formulation of atebrin in 1930 and chloro-quine in 1934. Although marshes, mosquitoes, poor living conditions, and malaria were associated repeatedly throughout history, it was not until 1880 that Laveran first observed parasites in the blood of fever patients and 1897 that Ross found malarial parasites in an Anopheles mosquito that previously had fed on a malaria patient. The following year, Ross worked out the complex life cycle of the malaria parasite using a Culex mosquito-sparrow malaria model. Shortly afterward Grassi and colleagues elucidated the life cycle of the human parasite and with Manson demonstrated that protection from mosquito bites provided protection from infection.
The now-confirmed relationship between malarial infection and mosquitoes led to expanded control efforts by chemically treating or reducing surface water where larval mosquitoes occurred. In 1936, the insecticidal properties of DDT were discovered by Muller and Weisman. DDT spraying was used in the successful eradication of introduced Anopheles gambiae mosquitoes from Brazil in 1939- 1940 and Egypt in 1942-1945. These successes and the eradication of malaria from the USA by spraying the inside walls of houses with DDT set the stage for the 14th World Health Assembly to adopt a global malaria eradication strategy that was implemented by the World Health Organization from 1957 through 1969. However, failure to sustain the efforts and funding after initial success, disregard for the magnitude of the malaria problem in Africa, and the onset of insecticide resistance in several of key vector species resulted in a global collapse of this effort that was followed by a general resurgence of malaria throughout tropical regions of the world. In 1992, the World Health Organization again selected malaria as the target disease for a global initiative to improve human health, and in 1998 launched the new “Roll Back Malaria” campaign to reduce malaria by 50% by the year 2010. Subsequent financial support by the U.S. Government under President Bush and the Bill and Melinda Gates Foundation has brought established and new technology into this campaign. Only time will determine if the human host finally will rid itself of the circumtropical malaria burden.
PARASITES AND LIFE CYCLE
Four species of human Plasmodium may be identified, in part, by clinical symptoms such as the pattern of fever and chills (Table I), morphology, and staining characteristics of the parasite within red blood cells, antigenic properties determined by serology, or genetic sequence. The Plasmodium life cycle is complex (Fig. 1). The female Anopheles becomes infected when gametocytes are ingested during blood feeding. Sexual union of gametocytes occurs in the mosquito midgut, after which the resulting ookinete penetrates the midgut wall and forms an oocyst. After asexual reproduction, the oocyst ruptures and the motile sporozoites make their way to the salivary glands. Humans become infected during blood feeding by the infective mosquito host when sporozoites are expectorated with mosquito saliva into the wound created by the mosquito bite. After entry into the circulatory system of the human host, sporozoites rapidly enter the liver where asexual reproduction occurs. Liberation of parasites from the liver may occur rapidly or be delayed, depending upon the species and strain of parasite (Table I). Once in the blood stream, parasites rapidly enter red blood cells where they multiply asexually. The synchronous liberation of parasites from the red blood cells results in rhythmic paroxysms characteristic of malarial disease. As the infection progresses, haploid gametocytes are formed in the peripheral blood stream from where they are ingested by blood-feeding mosquitoes, thereby completing the life cycle.
MOSQUITO VECTORS
Only female mosquitoes in the genus Anopheles serve as definitive hosts for the four species of human malarial parasites. Of these, species in the subgenus Cellia account for most of the current global transmission and include members of the Anopheles gambiae complex (gambiae, arabiensis) and An. funestus in sub-Saharan Africa, and the An. culicifacies complex, An. fluviatilis complex, An. stephensi, and An. minimus in Asia. Historically, the An. macu-lipennis complex was important in the Mediterranean and Europe, whereas species in other subgenera such as An. darlingi and An. albitaris have been responsible for the resurgence of malaria in South America.
EPIDEMIOLOGY AND DISEASE
Malaria remains a critical health problem of global proportions, causing an estimated 300-500 million clinical cases and more than one million deaths annually. It is a health problem of crisis proportions and a severe economic burden in 107 countries inhabited by 3 billion people (roughly 40% of the world population). The temporal concordance between crop growing and malaria transmission seasons frequently results in a serious loss of agricultural productivity. The distribution of malaria in time and space and the efficiency of transmission are limited by temperature requirements for the
TABLE ICharacteristics of Human Infection with Four Species of Plasmodium |
|||||
| Plasmodium species | |||||
| Characteristic | vivax | ovale | malariae | falciparum | |
| Incubation period (days) | 13 (12-17)a | 17(16-18) | 28(18-40) | 12 (9-14) | |
| Exo-erythrocytic cycleb | Present | Present | Absent | ||
| Merozoites/tissue schizont | > 10,000 | 15,000 | 2,000 | 40,000 | |
| Erythrocytic cycle (hours)c | 48 | 49-50 | 72 | ca. 48 | |
| Parasitemia (avg. per ml) | 20,000 | 9,000 | 6,000 | 20,000-500,000 | |
| Attack severity | Mild-severe | Mild | Mild | Severe | |
| Paroxysm duration (hours) | 8 – 12 | 8-12 | 8-10 | 16-36 or longer | |
| Relapses | + + | + + | + + + | None | |
| Period of recurrence | Long | Long | Very long | Short | |
| Duration of untreated infection (years) | 1.5-3 | 1.5-3 | 3^0 | 1-2 | |
Note. Modified from Bruce-Chwatt (1980). aStrain dependent, may be up to 9 months. bContinued production of merozoites within the liver.
cTime between red blood cell infection and rupture indicated by the pattern of paroxysms.
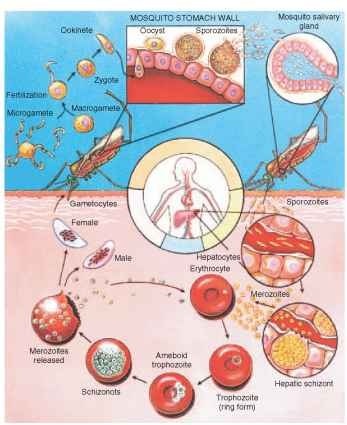
FIGURE 1 Generalized life cycle of the four human-infecting Plasmodium species.
development of the Plasmodium parasites within their poikilother-mic Anopheles hosts and the abundance, bionomics, and behavior of the different Anopheles vectors. P. vivax can develop at temperatures as low as 14.5°C and is found at more colder latitudes and higher elevations than P. falciparum that requires temperatures above 16° C (Fig. 2). In addition to ambient temperature, transmission efficiency depends almost entirely on Anopheles bionomics expressed as vec-torial capacity; species that are long-lived, rapidly develop parasites, and feed frequently on humans are the most efficient transmitters of malaria parasites.
The incubation period between infection and clinical illness varies among malarial species and strains, being shortest in P. falciparum and as long as 9 months in some northern strains of P. vivax ( Table I). Illness is characterized by the malarial paroxysm and, if untreated, increases in severity as the number of parasites multiply logarithmically. Typical complications include anemia and splenomegaly. In P. falciparum infection, changes in the structure of infected red blood cells create congestion and blockages within the circulatory system, causing coma (brain hemorrhages), jaundice, and ” black-water fever” with the passing of black urine (liver failure, nephritis), and severe dysentery (dehydration, renal failure). Infection during pregnancy frequently leads to abortion, stillbirths, and neonatal mortality. Some liver stages of P. vivax and P. ovale remain dormant and, if untreated, may relapse many years after the initial infection.
Infection imparts transient immunity that is maintained in endemic areas by almost constant re-infection. In hyperendemic areas, morbidity and mortality are highest among the non-immune, including travelers, infants, and pregnant women. Adults in these areas tolerate chronic infections and present a constant source of gametocytes for mosquito infection. Malaria mortality has selected for resistant and semi-resistant phenotypes from the genome of affected human populations, leading to the evolution and persistence of traits such as sickle
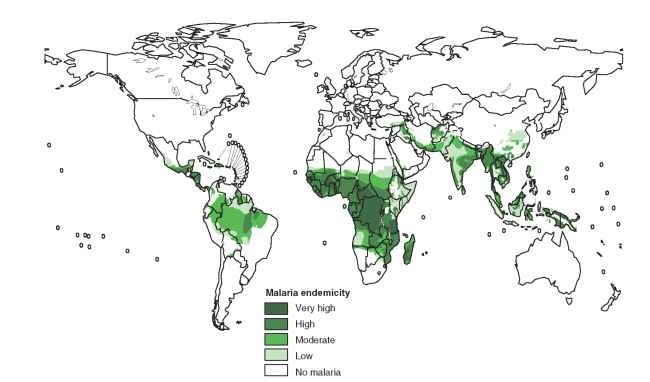
FIGURE 2 Global distribution of malaria.
cell anemia and Duffy blood group antigen that alter the structure and surface of red blood cells making them resistant to parasite infection.
TREATMENT AND CONTROL
Treatment has emphasized the use of chemical derivatives of the quinoline ring, originally found in quinine and present in chlo-roquine and primaquine. Primaquine has the important feature of destroying the liver stages of vivax and ovale, thereby eliminating relapses and chronic sources of parasite re-introduction. Resistance has led to the development of alternative drugs including proguanil, mefloquin, pyrimethamine, and sulphonamide; however, in some areas of Southeast Asia treatment of patients infected with multiple drug-resistant strains must revert to quinine with tetracycline. Extracts from plants in the genus Artemisia represent a new class of drugs from Asia that now are used for treatment in areas with widespread drug-resistant malaria.
Historically, public health efforts targeting eradication have combined active case detection and treatment with adult mosquito abatement. Active case detection has emphasized complete village-level surveys, the presumptive treatment of fever cases with chloroquine, and verification of malaria infection by slide examination. Residual house spraying with DDT and later malathion targeted indoor resting Anopheles females in an attempt to interrupt the transmission cycle. This combined approach resulted in remarkable successes in areas such as Sri Lanka, Pakistan, and India where the primary vector, An. culicifacies, rests almost exclusively within houses and cattle sheds. However, interest and funding to sustain successful programs waned and eventually collapsed. Recently eradication has changed to control, is limited to passive case detection and treatment, and has been incorporated into general village-level health programs. The recent Roll Back Malaria campaign has employed similar strategies but focused on pyrethroid-impregnated bednet and curtain distributions to interrupt transmission and Artemisia therapy to treat patients. These extramural funded programs have once again begun to reduce disease incidence.
I n addition to research to improve and expand the arsenal of drugs for patient therapy and the distribution of impregnated bed-nets and curtains, two new control approaches currently are being investigated: (1) vaccination (protection that probably will be of short duration and is expected within 7-10 years) and (2) genetic manipulation of vector competence in Anopheles (species-specific, costly, and untried). These research thrusts have been facilitated by the complete sequencing of the P. falciparum and the An. gambiae genomes that should enhance the understanding of transmission and provide new targets for intervention.
Success of malaria intervention in developing countries most likely will continue to be hindered by inadequate control delivery systems, political unrest, and the low socio-economic level of most rural populations that cannot sustain intervention. In the modern era, successes in malaria control typically have accompanied advances in education, economic well-being, and medical delivery systems that have led to sustainable programs.
