Juvenile hormones (JHs), acting in concert with ecdysteroids, I orchestrate the expression of larval-specific genes each time the insect molts to a new stage. These morphogenetic effects include determination of an immature body form and internal organs, hardness and color of the cuticle, and accompanying physiology and behaviors. At the conclusion of larval development, JH levels drop at critical times, allowing ecdysteroids to program expression of pupal and adult characteristics. JHs return in the adult stage, in which they have gonadotropic functions in connection with reproduction. In addition to their morphogenetic and gonadotropic actions, JHs are involved in dormancy and various types of polyphen-isms, including caste determination in social insects.
DISCOVERY
The discovery of JHs began in the 1930s, with a series of ingenious experiments conducted by Wigglesworth that were aimed at elucidating the hormonal control of molting. Trained as a medical doctor, Wigglesworth dedicated his life to basic studies of insect physiology, believing that knowledge gained would hold the keys to controlling insect vector-borne disease and agricultural pests. As a model experimental insect, Wigglesworth chose the Chagas’ disease vector Rhodnius prolixus, otherwise known as the ” kissing bug” because of its habit of sucking blood from the lips of sleeping humans. The choice of Rhodnius was inspired because its development is closely timed to its blood meals. This allowed Wigglesworth to precisely determine the physiological stage of the insects to coincide with his experimental manipulations. He found that 3 days after a blood meal, hormones are released into the Rhodnius system, stimulating the molt to the next stage. By performing a number of surgical procedures on the bug, Wigglesworth demonstrated the source and timing of hormone release. An advantage of working with insects as experimental animals is that they can survive for long periods without such seemingly vital organs as the brain, a fact that Wigglesworth exploited. He found that decapitation of animals prior to a 3-day critical period led to an arrest in development, even though the animal would remain alive for many months. If the brain was reimplanted, development resumed. He also found that the blood of a normally developing animal could reactivate development in the headless animal. This was achieved via a technique called parabiosis, in which the developmentally arrested animal was joined to the normal one by means of a tube that allowed blood from the two animals to mix. With these experiments, Wigglesworth demonstrated that hormones released from the brain trigger molting. This discovery actually had been made more than a decade earlier by Stefan Kopec, working with gypsy moth, but Wigglesworth’s experiments also revealed a new type of hormone, one that influenced the form taken by the animal after each molt.
Wigglesworth fundamentally changed the thinking about insect development, specifically the distinction between regulation of growth and regulation of form by separate hormones. Rhodnius passes through five nymphal stages before molting to the adult form. It is easy to distinguish the adults from the nymphs because of differences in pattern and color of the cuticle, as well as the fact that only adults have wings. Wigglesworth found that parabiosis of a fifth (last)-stage nymph with a young nymph prevented the former’s metamorphosis to the adult stage. Instead, the animal molted to a sixth-stage nymph, an extra immature stage that never occurs normally. A chemical in the blood of the young insect promoted continued expression of larval characters, and this factor came to be known as the “juvenile hormone.” The source of the JH was traced to a pair of small glands behind the brain called the corpora allata (Fig. 1 ). Surgical removal of the gland did not interfere with molting, but drastically altered the form taken after the molt, causing animals to become precocious adults. Reimplantation of the glands led to the return of larval characters.
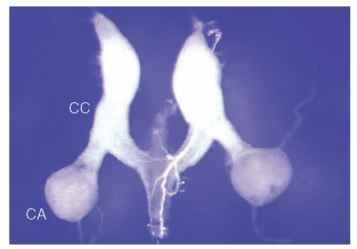
FIGURE 1 Photomicrograph of the corpora allata (CA), paired, spherical glands that are the sole source of the juvenile hormones in insects. Also shown are the elongated, white corpora cardiaca (CC). The CC and CA are positioned behind the brain, where they release hormones into the blood. Structures shown were dissected from the cockroach, Periplaneta americana.
While the corpora allata proved to be the sole source of JH, only very small amounts were available from the gland for chemical studies. The short supply of JH greatly constrained experimentation, slowing the process of discovery considerably. A breakthrough came with the discovery of large amounts of JH in abdomens of adult male silk moths by Carroll Williams. Ether extracts produced a dark orange material that he called the golden oil. Such an abundance of JH in a male adult at first was surprising, but already Wigglesworth had noted the essential gonadotropic role of JH associated with reproduction, that is, stimulation of egg and sperm development. The reason for enormous quantities of JH in male adult abdomens is probably its inclusion in spermatophores, which contain sperm together with nutritive and hormonal stores and are provided to the female during mating for fertilization and nutrition of developing eggs. Williams is credited with stimulating the modern era of JH research, by making available enough of the natural hormone to conduct biological experiments on its modes of action in many types of insects. This work also provided quantities of starting material sufficient for the eventual isolation and chemical identification of the hormone, which occurred in the late 1960s and early 1970s.
CHEMISTRY OF JUVENILE HORMONES
The JHs are lipophilic sesquiterpenoid derivatives of farnesoic acid. Their chemical nature came into focus in the early 1960s, beginning with observations that farnesoid components of beetle excreta had JH-like activity in bioassays. Although far less potent than the native hormone, these substances, including farnesol and its oxidized form farnesal, were suspected to be JH precursors. Subsequent synthesis of methyl farnesoate with an epoxide at position 10-11 by William Bowers in 1965 gave a highly potent compound. It is indeed ironic that this very same compound was discovered 8 years later to be JH-III, the most ubiquitous of the natural JHs (Fig. 2).
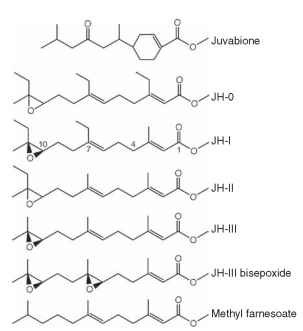
FIGURE 2 Structures of natural JHs and related compounds. Juvabione is the paper factor from North American balsam fir discovered by Williams, Slama, and Bowers. JH-I was the first natural JH discovered by Roller, and JH-II and JH-III soon followed. JH-III is present in most groups of insects, including the Lepidoptera. JH-0 is found in moth eggs, but its biological function is unclear. JH-0, JH-I, and JH-II are generally confined to the Lepidoptera. JH bisepox-ide, first isolated in Drosophila, has been found in other dipter-ans and ticks. Methyl farnesoate, a JH precursor, has been isolated from some insects and crustaceans, where it may serve as the active JH.
Another interesting prologue to the discovery of the natural JHs was the discovery of a curious “paper factor” by Karel Slama and Carroll Williams in the mid-1960s. Slama had carried insects from his laboratory in Czechoslovakia to the laboratory of Williams at Harvard University for a series of joint experiments. Some weeks after arriving, some insects began to develop extra stages and many died. Nothing of the sort was noticed in Czechoslovakia. It was eventually determined that the paper towels used to line the containers holding the insects contained compounds with JH-like biological activity. The substances were absorbed through the insect cuticle upon contact with the paper towels. The paper factor turned out to be a mixture of terpenoids in the wood pulp from which the paper towels were manufactured. These compounds were found only in American and Canadian balsam fir and not in European trees. One of these substances was chemically identified by Bowers and colleagues as “juvabione” (Fig. 2), which had a structure reminiscent of farnesol.
These studies provided new information on two critical issues of the time. First was the question of JH structure. Observations that farnesol and methyl farnesoate, along with juvabione, possessed JH-like biological activity made it likely that terpenoid chemistry was involved. This guided further attempts to chemically define the natural material(s). The second issue was whether JHs or analogs such as juvabione could be used to produce, as Carroll Williams suggested, a new class of “third-generation” pesticides. Juvabione constituted a relatively stable terpenoid with potent insecticidal activity and hence stimulated further interest in this novel concept for new insecticides that would be both highly insect-selective and safe for warm-blooded animals.
With this as background, Roller and colleagues isolated sufficient quantities of JH from silk moth abdomens for identification of the first natural JH in 1967. The carbon skeleton was identified as a 15-carbon sesquiterpene substituted at positions 7 and 11 with ethyl groups. Further key structural features were the presence of a methyl ester and an epoxide at carbons 10 and 11, respectively. This landmark achievement was followed rapidly by the discovery of a second JH, also from moths. These molecules were named JH-I and JH-II, respectively, differing only at carbon 7, which was ethyl-ated in JH-I and methylated in JH-II (Fig. 2) . These two JHs are largely restricted to the Lepidoptera. Within a few years, a third hormone was identified by a completely different approach. In this instance, corpora allata from the moth Manduca sexta were removed and placed in organ culture containing a radiolabeled methyl donor, 14C-labeled methionine. The corpora allata incorporated the 14C-methyl group into the ester moiety of JH. This isotope-labeled synthetic product was isolated and identified as JH-III. It is the most cosmopolitan of JHs, occurring in most insect groups, including the Lepidoptera. It has methyl groups at positions 7 and 11 (Fig. 2). Three additional JH structures have been identified: JH-0 and 4-methyl JH from moth eggs and JH-bisepoxide first found in the fruit fly Drosophila and now known to occur in other dipterans as well as ticks (Fig. 2).
The JHs are derived from acetyl CoA and/or propionyl CoA via mevalonic acid and homomevalonic acid in the sterol biosyn-thetic pathway. The final steps of JH-III biosynthesis go by way of farnesol^farnesoic acid^methyl farnesoate, to which an epoxide is formed at carbons 10-11. Owing to their low aqueous solubility, the JHs are transported through the blood via binding proteins upon their release from the corpora allata. These binding proteins also protect JH from degradative enzymes.
In some insects, the corpora allata synthesize a precursor of the biologically active form of JH, which is converted to the active form in target tissues. For example, silk moth adults produce JH acid in the corpora allata and convert it to JH-I in the accessory glands of the abdomen. It is also known that the ovaries of certain species of mosquito can synthesize JH from precursors under culture conditions. Whether this occurs under natural conditions i n vivo has not been demonstrated.
The levels of JH in the blood are regulated through a combination of synthesis and degradation. Synthesis by the corpora allata is promoted by neuropeptides called allatotropins and suppressed by alla-tostatins. The first allatotropin (Manse-AT) was identified from the moth Manduca sexta. Surprisingly, the peptide proved to be active in the adult stage of this moth, but not in immature stages. Recent evidence however indicates that Manse-AT is active in other insect species, including immature stages. A variety of allatostatins have been characterized. These are neuropeptides synthesized in brain neurons that project to the corpora allata. Their release from nerve endings in the gland inhibits the synthesis of JH. It is also possible that circulating allatostatins help to suppress JH synthesis when appropriate.
The removal of JHs already in the blood, a necessary condition for metamorphosis, occurs through two enzymatic degradation pathways. One is through cleavage of the ester bond by JH esterase, and the other through epoxide destruction by epoxide hydrolases.
BIOLOGICAL ACTIONS
Morphogenetic Effects
The presence of JHs in the blood promotes expression of juvenile characters, chief among these being an immature body form or morphology. For insects such as grasshoppers, which undergo incomplete metamorphosis, the effects are not so visible outwardly. The early stages look like miniature adults except for the absence of wings, but they also lack functional reproductive organs. However, for those insects such as flies, moths, and bees, which undergo complete metamorphosis, the effects are extreme. The immature stages are wormlike with no wings, legs, compound eyes, antennae, or other adult structures.
Determination of holometabolous immature or larval body plan by JH represents its morphogenetic action. The decision to develop larval characters during development is made near the end of each larval stage, when ecdysteroid levels increase to initiate the molt. Elevation of ecdysteroids causes a cessation of feeding and a new round of gene expression appropriate for the next stage of development. If JHs are present at this time, genes for larval characters are expressed whereas genes appropriate for pupal or adult characters are repressed. A primary larval character is the type of cuticle secreted by the epidermal cells. Larval cuticle is lighter and more flexible than pupal or adult cuticle, which are characteristics resulting from expression of larval cuticle protein genes that predominate under the influence of JHs. The flexibility of larval cuticle has to do with the absence of cross-linking between proteins and between proteins and chitin, the latter constituting the polysaccharide component of the cuticle. In contrast, pupal and adult cuticles are hard and dark, indicating a high degree of sclerotization and melanization.
Specification of pupal or adult features by JH, or lack thereof, is associated with transient, hormone-sensitive periods during development. It is important to note that the actions of JH depend not only on its presence in the blood, but also on the ability of cellular targets to respond. This latter condition presumably reflects the presence of suitable receptors required to mediate the action of the hormone. It has been observed in many studies that JH responsiveness occurs after priming, which could be associated with expression of receptor genes or other molecules necessary to complete the signaling pathway. For example, in moths, specification of a wandering period in preparation for pupation occurs upon the appearance of ecdysteroid peaks in the complete absence of JH. These “commitment” peaks of ecdysteroid prime the system to respond later in the same stage to elevated JH levels that occur in concert with ecdysteroids, which specify pupal features.
The morphogenetic actions of JH during juvenile stages have historically been inextricably linked with those of ecdysteroids. In this context, the role of JH is to ensure that ecdysteroids program larval phenotype. Nevertheless, JH appears to act alone in promoting the proliferation of imaginal disc tissues, while at the same time suppressing differentiation. The ability of discs to differentiate depends on exposure to ecdysteroids in the absence of JH, which occurs during the last larval instar. Furthermore, intriguing new findings by Truman and colleagues show that JH and nutrient-dependent signals, perhaps insulin-like hormones, regulate the growth and differentiation of imaginal disc primordia. In this way, JH helps to program proper scaling of tissues leading to normal-sized adults.
In many insects, considerable development of the gonads takes place during the larval stages. This is especially true for insects such as the silkworm, Bombyx mori, which does not feed during the adult stage. Within hours of emergence, these animals mate and lay eggs.
The ability to mate and produce viable eggs so soon after emergence means that gonadal development is well along during the larval and pupal stages. JHs promote the development of gonads and gametes during the immature stages, but must disappear in order for final developmental steps to be completed. This drop in JH levels just prior to the pupal stage therefore serves both morphogenetic and gonadotropic functions.
Effects of JH in the Adult Stage
The decrease in JH levels just prior to metamorphosis is only a temporary condition. The corpora allata are retained in the adult stage, and JH eventually reappears to regulate adult reproductive functions. JH promotes sperm and egg development and hence is said to have “gonadotropic” functions. In the female, JH directly promotes the synthesis of lipo- and glycoproteins in the fat body and their uptake into the developing oocyte. This process, called vitellogenesis, is a complex process of yolk deposition under the control of hormones and nuclear transcription factors. In many insects, JH levels rise and fall in a cyclic fashion as discrete batches of oocytes go through the vitellogenic process.
In other instances, as in some mosquitos, JH exposure leads to “competence” of the fat body to synthesize vitellogenic proteins upon later exposure to ecdysteroids. Likewise, JH exposure is required to induce competence of the ovaries to respond later to peptide hormones from the nervous system, thus stimulating uptake of vitellogenic proteins. In these instances, the gonadotropic actions of JH appear to be priming steps in preparation for ecdysteroid action.
Gonadotropic functions of JH in the male have to do with growth of the sperm. Sperm growth requires JH in many insects. However, maturation from spermatocytes to motile spermatozoa requires a drop in JH. Thus, as observed for oocyte development, JH exerts both positive and negative influences in sperm development.
Polyphenism and Caste Determination
Many insects have the remarkable ability to develop into alternate forms as they become adults. These alternate forms together with accompanying physiology and behavior, referred to as polyphen-ism, do not reflect differences in the genetic makeup of individuals. Rather, they result from a particular pattern of gene expression under hormonal control. Most polyphenisms are controlled by JHs acting at certain sensitive periods during immature development.
Some of the most common instances are caste polyphenisms observed in social insects such as bees, ants, and termites. In these insect societies, larvae can develop into workers, soldiers, or queens, depending on the diet they are raised on and the hormonal levels that result. If bee larvae are reared in a special cell in the hive and consistently fed a nutritious “royal jelly” beginning during the third instar, they develop into queens. Treatment with JH will mimic this effect. If this feeding is delayed, larvae develop into workers instead. In certain ants, development of queens is regulated during embryonic development by JH levels. During postembryonic development, larvae fed a high-protein diet produce large amounts of JH, bringing blood levels to a threshold necessary for specification of soldier phenotype. If larvae are fed a diet lower in protein, JH levels are correspondingly lower and development to worker is specified. The number of soldiers in the colony is also determined by a soldier-inhibiting pheromone, which elevates the JH threshold for soldier specification. Alternative body forms and behaviors in insect colonies provide for cooperative functions between members of the society to serve the greater whole.
Many types of phase polyphenisms occur in nonsocial insects. For example, locusts occur either in solitary or in migratory phases, depending on population density. Differences in both behavior and physiology are characteristic of these phases. Solitary locusts are sedentary, pale green, yellow, or brown, and have short wings and large ovaries. Crowding causes the switch to the gregarious phase, in which individuals are brightly colored, have longer wings and smaller ovaries, and are easily induced to engage in long flights. Both JH and peptide neurosecretory hormones from the brain are involved in the determination of these two phases.
Aphids exhibit at least two different types of phase polyphenism as a response to seasonal conditions: food quality and crowding. In one type, adults switch between winged or apterous (no wings) forms. The other type has to do with the mode of reproduction, either sexual or parthenogenetic. During the longer days of spring and summer, apterous, parthenogenetic females predominate, and JH is involved in specification of these forms. As winter approaches, winged forms are produced, allowing for dispersal. Later, in autumn, males and females mate and lay eggs, which overwinter and hatch in the spring. In this context, body forms and accompanying dispersal or migratory behaviors maximize survival as the season changes.
In summary, JH and other neurosecretory hormones are important determinants of polyphenisms, which result in different body forms, reproductive physiologies, and behaviors in the adult stage. It is emphasized that such variability of form and function is not the result of genetic differences between individuals, which would be classified as polymorphisms. Rather, insects have the enormous potential to change form in response to environmental conditions through hormonal control mechanisms. Depending on the needs of a social colony, or changes of season and in food availability, the complex endocrinology of insects enables them to assume various alter egos to enhance success and survival.
Behavioral Effects of JH
The presence or absence of JHs has profound effects on behavior, some of which have been mentioned in the preceding text. Throughout the stages of immature development, JHs program gene expression in the nervous system for the expression of behaviors appropriate for juvenile life, including, for example, locomotion, host or prey seeking, feeding, and silk spinning. From the point of view of behavior, the larva is an animal completely different from its later adult form.
In moths, the disappearance of JH at the end of the last instar allows ecdysteroids to program new behaviors appropriate for metamorphosis. Insects stop feeding, void their guts, and engage in wandering behaviors to locate a suitable pupation site. Once this is accomplished, a series of behaviors leads to silk spinning for cocoon construction.
Upon becoming adults, female mosquitos initiate the search for a blood meal and become sexually receptive to males only after release of JH into the blood. In milkweed bugs, JH levels are influenced by day length, temperature, and food quality. Under short day lengths, JH levels drop and insects engage in migratory behavior immediately after molting to the adult stage. However, long days and warm conditions lead to high JH levels, whereby flight is inhibited and reproduction ensues.
Grasshopper females that have had corpora allata removed rebuff male sexual advances until JH is reintroduced by injection.
In crickets, the male sings a species-specific calling song to attract the female for mating. The female responsiveness to this song is enhanced by elevated JH levels. These examples serve to illustrate the dramatic effects that JH has on the behavior of insects, effects that are specific and appropriate for each particular life stage.
Dormancy—Diapause
Insects are able to enter prolonged states of dormancy referred to as diapause, allowing them to survive freezing and low food supplies during the winter. Diapause can occur at any stage (egg, larva, pupa, or adult) and is triggered by decreasing day length, low temperatures, decreased food or food quality, or a combination of these factors. The insect response to these environmental factors is mediated by a variety of hormones, depending on the stage and species.
Adult diapause is largely synonymous with reproductive diapause. Beetles, butterflies, and flies enter a reproductive diapause when the brain inhibits synthesis of JHs by the corpora allata. The lack of JHs leads to both physiological and behavioral changes, including cessation of vitellogenesis, loss of flight muscle, increasing stores of lipid in the fat body, burrowing, and construction of hibernacula (overwintering chambers). Implantation of corpora allata or injection of JHs reverses reproductive diapause.
J H involvement in larval diapause also has been documented. The southwestern corn borer Diatraea grandiosella enters diapause during the last instar when JH levels are depressed but are still high enough to inhibit development to the pupal stage. The animal spins a hibernaculum, exhibits a light pigmentation, and actually undergoes several “stationary” molts. Diapause in this stage lasts as long as JH levels remain elevated.
MOLECULAR BASIS OF ACTION
It is presumed that JHs exert their effects through receptor activation. Until recently it was a curious and surprising fact that, almost 70 years after the biological activity of JH was described by Wigglesworth, no definitive JH receptors had been identified. There are now tantalizing indications that candidate JH receptors are coming into greater focus. One of these involves a nuclear receptor called ultraspiracle, or USP, a protein that regulates gene expression by forming a dimer complex with ecdysteroid receptors. The complex then binds to regulatory sequences on genes to turn them on or off. Grace Jones and Alan Sharp have demonstrated that JHs bind specifically to USP, although the affinity for this binding is lower than is generally expected for hormone-receptor interactions. It is proposed that JH binding to USP may influence how it interacts with ecdys-teroid receptors to regulate gene expression. The influence of JH and USP on EcR actions would seem to be a very plausible scenario for joint actions of JH and ecdysteroids, but further work is needed before USP can be confirmed as a JH receptor.
Although early accounts of JHs focused on their uniqueness with respect to insect biology, the elusiveness of JH receptors has prompted a closer look at possible similarities between signaling mechanisms common to insects and mammals. Indeed, the chemical structure of JHs resembles those of retinoids and farnesoids, both of which function in mammalian nuclear signaling by activating retinoic acid receptors, retinoid X receptors, and the farnesoid X receptor. JH and farnesoids are capable of activating some of these receptors, and some retinoids are known to have JH-like activity. It also has been observed that vertebrate thyroid hormones mimic some of the actions of JHs. Efforts are under way to identify receptors homologous to their mammalian counterparts as possible JH receptors.
Recently, promising new genetic approaches have been employed in searches for JH receptors. A strain of fruit flies has been identified that is resistant to the toxic actions of methoprene, an insecticidal JH analog (see next section for details). The resistant flies have a defect in a gene that encodes MET, a protein related to the vertebrate aryl hydrocarbon receptor (AHR), which, upon binding to a diverse range of hydrocarbons and xenobiotics, activates a battery of genes involved in their metabolism. If the MET protein has properties similar to AHR, this might help explain why many synthetic chemicals such as fenoxycarb and pyriproxyfen have very potent JH-like effects, but bear little obvious structural similarity to natural JHs. Paradoxically, fly lines carrying the defective MET gene show no developmental problems, which would be expected if JH signaling is compromised. This has caused considerable doubt about the possible role of MET as a JH receptor. However, recent work by Marek Jindra and colleagues has shown that silencing of the MET ortholog in the red flour beetle, Tribolium castaneum, not only confers resistance to metho-prene but also leads to developmental problems consistent with deficits in JH signaling. Further work is needed to fully characterize this promising new candidate JH receptor.
JUVENILE HORMONES AND
INSECT CONTROL
The discovery of JHs in the late 1960s by Roller and others stimulated a period of great excitement regarding the concept of third-generation pesticides foreseen by Carroll Williams in the early 1960s. It was known that JHs and related substances such as juvabi-one could disrupt insect development with lethal effects. Likewise, surgical removal of the corpora allata led to precocious metamorphosis, also with lethal effects. It therefore seemed that insect hormones or their analogs could be synthesized and used to accomplish a form of “birth control” for insects.
This idea occurred to Carl Djerassi and Alejandro Zaffaroni, two former colleagues at the Syntex Corporation, who were involved in synthesis of the progestin norethindrone that led to the oral contraceptives for humans. They formed a new company called Zoecon, a name chosen to denote “animal control” through the use of hormones and related chemical analogs. Their principal objective was to develop insect hormones for use as birth control agents specific for this group of animals. Chemists at Zoecon soon produced analogs of JH called “juvenoids” that were much more stable and could penetrate the cuticle. One of the first of these analogs to be granted a registration from the Environmental Protection Agency was meth-oprene (Fig. 3), a compound with outstanding biological activity against mosquitos, fleas, and biting flies. By mimicking JH, metho-prene prevents treated insects from completing metamorphosis, and insects die during the pupal stage. Other juvenoids such as hydro-prene (Fig. 3) are more effective against insects with incomplete metamorphosis, such as cockroaches. Treated cockroaches actually reach the adult stage, but the presence of juvenoid during the transition to the adult results in only a partial adult phenotype in which many adult features are abnormal. For instance, the gonads are not fully developed, leading to sterility, and crinkled wings are an obvious morphological defect.
Subsequent efforts by several agrochemical companies have generated a variety of juvenoids, many with potent JH-like biological activity but with aryl rings substituted for isoprenoid units and without obvious similarities to natural JHs. These include fenoxycarb and pyriprox-yfen (Fig. 3). Juvenoids have proved to be commercially successful for
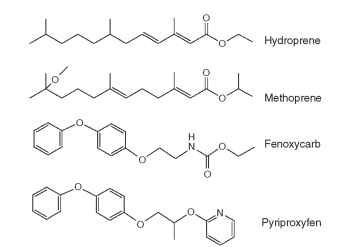
FIGURE 3 Structures of synthetic JH analogs, commonly called juvenoids. Methoprene has been useful in the control of mosquitos, fleas, and biting flies, while hydroprene was developed for cockroach control in dwellings. Fenoxycarb and pyriproxyfen, heterocyclic compounds with little resemblance to JHs, nevertheless have potent JH-like biological activity against a wide range of insects.
insects that are pests in the adult stage. However, because they do not control insects in the immature stages, they have not proved useful for large-scale agricultural pest control. For this purpose, JH antagonists are needed for induction of precocious metamorphosis. So far, that goal remains as elusive as the search for the JH receptor.
