Hyperparasitism is a highly evolved behavior in the Hymenoptera and in a few species of Diptera and Coleoptera, in which an adult hyperparasitoid (or secondary parasitoid) oviposits on or in a primary parasitoid host that has attacked another (usually herbivorous) insect species. The larval offspring of the hyperparasi-toid cause the death of the primary parasitoid. Ecologists emphasize this interaction as a food-web “community.” This article focuses on the hymenopteran microwasps in which hyperparasitism occurs.
There are a variety of behaviors of hyperparasitoids depending on the species of secondary and primary microwasp parasitoids, which in turn are influenced by the species of phytophagous host, often an insect pest. In addition, there is an economic interest in hyperpara-sitism because if primary parasitoids are considered to be beneficial insects when used in biological control programs, it would seem that hyperparasitoids that attack primary parasitoids would be detrimental. However, hyperparasitoids may play a positive role by preventing extreme oscillations of the primary parasitoids that might reduce the numbers of the phytophagous host enough to cause the local elimination of both the insect pest and the beneficial primary parasitoid.
EVOLUTION
Hyperparasitism has evolved in only three insect orders: in Hymenoptera (in 17 families) and in a few species of Diptera and Coleoptera. Its evolution was preceded by that of primary parasitism that evolved in the Hymenoptera during the Jurassic period, about 135 mya. In the primary parasitoids, ectophagous feeding probably evolved before endophagous, with the parasitoid egg deposited near or on the host rather than in it. Hence, ectophagous parasitoids usually attacked concealed hosts, often within galleries in wood or plant galls. The use of venom by primary parasitoids apparently developed very early and produced physiological changes in the host. Although the venom of the more ancestral ectophagous parasitoids resulted in idiobiosis (permanent paralysis or death), the venom of the specialized endophagous species tended toward koinobiosis (temporary or nonlethal paralysis).
Facultative hyperparasitism probably evolved from primary ectophagous parasitoids because few special adaptations are needed to oviposit and feed externally on a primary parasitoid as well as on the primary parasitoid’s phytophagous host. Obligate hyperparasit-ism has a wider taxonomic distribution and may have evolved via facultative hyperparasitism as an opportunistic behavior to specialize only in attacking readily available primary parasitoid hosts—especially if they share similar physiological and/or ecological attributes. Hence, it is not surprising that hyperparasitoid species can be either ecto- or endophagous, whereas some are idiobionts and others are koinobionts.
The host spectrum of hyperparasitoids is broader at the species level than that of primary parasitoids, but hyperparasitism is usually restricted to immature stages of hymenopteran hosts (larvae and/or pupae) that are natural enemies mainly of phytophagous insects in the Hemiptera (mainly suborder Sternorrhyncha), Lepidoptera, and the hymenopteran suborder Symphyta. Hyperparasitoids rarely attack the egg and adult stages of primary parasitoids. Also interesting is that some families of Hymenoptera that are well known for their species of primary parasitoids (Braconidae, Trichogrammatidae, Aphidiidae, Mymaridae, and almost the entire superfamily Proctotrupoidea) do not seem to have evolved any hyperparasitoids. Similarly, in the order Diptera, hyperparasitoids are absent in some important parasitic groups such as the family Tachinidae.
MULTITROPHIC ECOLOGY
There are two complementary ways that ecologists look at the interacting food-web community involving hyperparasitoids. One aspect is the “bottom-up” effect beginning with the first trophic level that shows both inter- and intraspecific plant variations influencing the ecology and behavior of the second trophic level of phytophagous (herbivorous) insects. This in turn is one of the fundamental determinants of the third trophic level of entomophagous (carnivorous) insects such as the primary parasitoid microwasps. Finally, insect hyperparasitism is the highly evolved fourth trophic level, wherein a secondary parasitoid microwasp oviposits on or in the primary parasitoid and kills it. The other complementary aspect is the “top-down” view by which the hyperparasitoid at the fourth trophic level exerts selective pressure on the primary parasitoid at the third trophic level. The next interaction is with the phytophagous insect at the second trophic level that in turn feeds on the plant at the first trophic level. This food-web community can have economic importance if the plant is an agricultural food crop or even a forest used for commercial lumbering or as a park.
APHID COMPLEX
Over many decades, studies of hyperparasitism have been conducted on the primary parasitoid microwasps in the Hymenoptera that attack the Hemiptera in the suborder Sternorrhyncha, and in particular the superfamily Aphidoidea, with special emphasis on the family Aphididae. The aphid-primary parasitoid-hyperparasitoid food web has been used as a model system in community ecology partly because of the economic importance of aphids as worldwide pests on a variety of agricultural crops and forests, but also because of the relative ease of rearing aphids, their primary parasitoids, and hyperparasitoids in the laboratory and/or greenhouse for precise behavioral and ecological studies. Primary aphid parasitoids are found in only two families of Hymenoptera: all genera of the Aphidiidae (Braconidae: Aphidiinae) and the Aphelinidae (Aphelinus and related genera). These primary parasitoids of aphids are in turn attacked by many genera of hyperparasitoids in three hymenopteran superfamilies: Chalcidoidea (Pteromalidae: Asaphes; Encyrtidae: Syrphophagus; Eulophidae: Tetrastichus), Cynipoidea (Alloxystidae: Alloxysta), and Ceraphronoidea (Megaspilidae: Dendrocerus).
Aphid hyperparasitoids can be divided into two categories based on adult ovipositional and larval feeding behaviors:
1. The female wasp of endophagous species such as Alloxysta (Charips) victrix (Fig. 1A) deposits her egg inside the primary parasitoid larva while it is still feeding on and developing inside the live aphid, but before the aphid has become mummified (a mummy is the hardened exoskeleton of the dead aphid that remains attached to the leaf). Being a koinobiont hyperparasitoid, the larva usually does not hatch until after the mummy has been formed by the primary parasitoid larva. Then the hyperparasitic larva feeds internally on the primary parasitoid larval host.
2. The female wasp of ectophagous species such as Asaphes lucens (Fig. 1B) and Dendrocerus (Lygocerus) carpenteri (Fig. 1C) deposits her egg on the surface of the primary parasitoid larva after the aphid has been killed and mummified. To do this, the female must first drill a hole in the mummy in which to deposit her egg. Then the hyperparasitic larva feeds externally on the primary parasitoid larva while both are still inside the mummy. The venom of species varies such that Asaphes is an idiobiont, whereas Dendrocerus is a koinobiont. An unusual species is Syrphophagus (Aphidencyrtus) aphidivorus, whose females display a “dual ovi-positional” behavior by attacking primary parasitoid larvae both within living aphids (Fig. 1D) and inside dead aphid mummies (Fig. 1E). Either way, S. aphidivorus develops as an endophagous koinobiont hyperparasitoid.
However, in all species of aphid hyperparasitoids, further development is similar to that of primary parasitoids, such that pupation is also inside the dead aphid mummy. Then the single adult hyperpara-sitoid emerges by cutting a hole in the dorsum of the mummy. After pulling itself out, the new adult male or female is ready for mating. From the time of the female’s attack on the primary parasitoid larva/ pupa, the period of hyperparasitoid development from egg deposition to adult emergence varies with different hyperparasitoid species from as short as 16 days to 25 days or more.
NONAPHID COMPLEX
Hyperparasitism exists not only with aphids but also with almost all insect taxa. There is a complex of primary and secondary parasi-toids associated with other members of the suborder Sternorrhyncha (scale insects, whiteflies, mealybugs, psyllids), as well as with various orders such as the Lepidoptera (gypsy moth, leafminers, budworms, stem borers), Diptera (gall makers, leafminers), Hymenoptera (cynipid gall makers, leafcutter bees, sawflies), and Coleoptera (lady beetles, weevils).
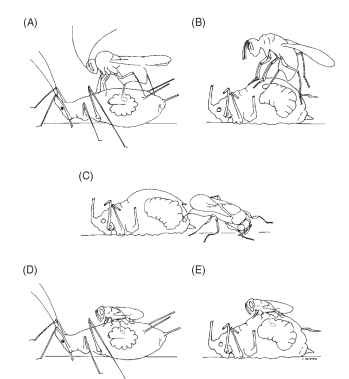
FIGURE 1 Female ovipositional behavior of four genera of aphid hyperparasitoids. (A) Endophagous koinobiont Alloxysta victrix jumps on a live parasitized aphid and deposits her egg internally inside the primary parasitoid microwasp larva while the aphid is still alive, but before mummy formation. (B) Ectophagous idiobiont Asaphes lucens stands on top of a dead aphid mummy, drills a hole, and deposits her egg externally on the surface of the primary para-sitoid larva developing inside the mummy. (C) Ectophagous koino-biont Dendrocerus carpenteri stands on the leaf, backs into the dead aphid mummy, drills a hole, and deposits her egg externally on the surface of the primary parasitoid larva developing inside the mummy. (D and E) ” Dual ovipositional” behavior of endophagous koinobiont Syrphophagus aphidivorus. (D) Syrphophagus stands on top of a live parasitized aphid and deposits her egg internally inside the primary parasitoid larva while the aphid is still alive, but before mummy formation. (E) Syrphophagus stands on top of a dead aphid mummy, drills a hole, and deposits her egg internally inside the primary parasitoid larva developing inside the mummy. [Reprinted from Sullivan, D. J. (1988). Aphid hyperparasites. In “Aphids, Their Biology, Natural Enemies and Control” (A. K. Minks and P. Harrewijn, eds.)
BIOLOGICAL CONTROL
Biological control uses predators, parasitoids, and pathogens to reduce an insect pest’s population to an acceptable level. Because primary parasitoids are considered beneficial, hyperparasitoids may interfere with such programs. It has been the policy in biological control to exclude “exotic” (nonindigenous) obligate hyperparasitoids by enforcing quarantine procedures during importation. It is less easy to decide what to do with ” exotic ” facultative hyperparasitoids when no exclusively primary parasitoids are available, and so perhaps admitting these could be beneficial. Such dilemmas are evaluated with caution, and the solution depends on the seriousness of the insect pest problem. On the other hand, “indigenous” hyperparasitoids (whether obligate or facultative) already exist in the ecosystem and may or may not interfere with the beneficial “exotic” primary parasitoid.
CONCLUSIONS
Hyperparasitism intrigues entomologists because of its multi-disciplinary relationship to evolution, ecology, behavior, biological control, taxonomy, and mathematical models. More field studies are needed to determine whether hyperparasitoids are always detrimental to biological control programs. Perhaps, instead, they could have a beneficial influence by regulating the extreme/detrimental population oscillations of the beneficial primary parasitoids.
