Tremendous progress has been made in the development of genetic engineering technologies in insects. This article empha-_JL_ sizes studies with the vinegar fly, Drosophila melanogaster, because, as a result of its successful use as a genetic model for eukary-otic genetic systems, developments in genetic engineering with this species establish a benchmark for what can, or could, be done in other insect species. The article also discusses the more recent use of transposable-element-based genetic transformation procedures in nondrosophilid insects and concludes that many of the tools required for genetic manipulations of nondrosophilid insects are now available.
The term “genetic engineering” is typically taken to refer to the direct manipulation of genes. It has become synonymous with a more general term, ” DNA technology, ” which has come to encompass all contemporary molecular-based techniques. However, many insect geneticists were using “DNA technology” before the development of recombinant DNA technology in the 1970s and 1980s. Genetic control approaches applied to such insect pests as the Mediterranean fruit fly (Ceratitis capitata) (medfly), the mosquito (Culex tarsalis) , and the Australian sheep blowfly (Lucilia cuprina) used genetics to develop new strains that could be used in insect control and/or eradication programs. The tools of these pioneers were not DNA modification and restriction enzymes, thermocyclers, or DNA sequencers, but rather radiation sources, microscopes, and the knowledge that mutations and chromosomal rearrangements could be created and selected for. These tools have now been surpassed, but one aim remains the same: the generation and application of new genetic strains of insects that can be used to control pest insect species. The development of sophisticated genetic tools, in conjunction with the rapid progress being made in genomics, will provide insect scientists with the ability to characterize and manipulate, in hitherto unimaginable ways, insect genes.
GENETIC ENGINEERING IN DBOSOPHILA MELANOGASTER
Genetic Technologies are More Advanced in Drosophila than in Other Insect Species
One cannot discuss the genetic manipulation of insects without describing the molecular genetic tools that are available in D. mela-nogaster. Traditionally, a gulf has existed between entomologists
who view the harmless vinegar fly as being distant to the problems of insect control and Drosophila geneticists who utilize the many biological attributes of Drosophila to understand the basis of gene action. This gulf will close as comparative genomics reveals similarities and differences in the conservation of many genes and molecular pathways between Drosophila and other insect species. The power of this comparative approach to modern biology will offer insect scientists and traditional entomologists exciting opportunities to bring the power of genetics and molecular biology to the control of insects. The development and application of these tools is what insect scientists seek to achieve in pestiferous and beneficial insects.
Genetic engineering in D. melanogaster is an extremely mature technology. It is founded on several independent phenomena:
1. The presence of a transposable element, called the P element, which is an efficient genetic transformation vector. This vector has been available and exploited since the early 1980s.
2. The ability to create and maintain genetic mutants by traditional techniques such as chemical- or radiation-induced mutagenesis or by transposon insertion mutagenesis, and the construction and availability of balancer chromosomes to maintain many of these mutants.
3. The presence of strains that lack the P element, thus providing recipient strains suitable for P element transformation.
4. The completion of the Drosophila genome project and the public availability of the data generated.
These planks of achievement are a consequence of the intense and sustained research that has been invested into Drosophila over the course of the last 90 years. The picture in all other insect species is, by comparison, sparse. For example, transposable elements capable of transforming nondrosophilid species have been available only since 1996. Also, traditional mutagenesis approaches have been used to generate mutations in a handful of insect species. Many of these have been lost because of problems arising from the rearing of these species (it should be noted that what attracted T. H. Morgan to Drosophila was the ease with which it could be reared and mated in the laboratory) and, often, because it had been necessary to depend on a handful of dedicated workers to maintain these strains. (In Drosophila, by contrast, there are central repositories for strain maintenance as well as hundreds, if not thousands, of researchers who maintain even the most problematic genetic stocks.) Except for medfly, balancer chromosomes have not been constructed in nondrososphild insects.
Two other factors are important. The interactions, if any, of the transposable elements so far known to transform nondrosophilids with components of the insect genome remain unknown, as do the molecular mechanisms by which these elements move both within and between insect genomes. Second, to date, no insect species other than Drosophila has had its entire genome sequenced. Some mosquito genomes are the target for current and future genomic projects.
Transformation Technologies in D. melanogaster
P ELEMENT TRANSFORMATION
Population geneticists in the 1970s had observed that when males from certain strains of flies recently established from wild populations were mated to females from long-established laboratory populations, a number of abnormal traits were observed in the progeny. These traits included high rates of mutation, sterility, and recombination in males, traits that are not usually seen in this species. Collectively, the traits that arose only when specific hybrid insects were created were thought to be manifestations of a single syndrome that became known as “hybrid dysgenesis.” Because the factors responsible for this syndrome were transmitted by males, they were referred to as paternal or “P” factors. Those working on P-factor-mediated hybrid dysgenesis quickly realized that there were multiple factors that mapped to many different locations within the genome. Some of the mutations that were induced during hybrid dysgenesis were very unstable and were themselves capable of mutating further to result in more extreme phenotypes or to revert to wild type. This instability as well as other genetic observations suggested that the P factors were mobile genetic elements or transposons. As long ago as 1989, Engels gave a comprehensive description of the P element, P factors, and their use in genetic transformation.
Concurrent with the efforts of population geneticists to understand the phenomenon of hybrid dysgenesis were efforts of molecular geneticists to clone genes from D. melanogaster. The eye color gene known as white was one of the first genes to be cloned from this species, largely owing to the great amount of genetic analysis that had been done on this locus. Having the white gene cloned, provided a unique opportunity to isolate P factors. Because P factors were responsible for causing mutations, a genetic screen was performed to seek mutations induced by hybrid dysgenesis of the white gene. The reasoning behind this experiment was that once a P-factor-induced mutation of the white gene was obtained, it should, in theory, be readily cloned by conventional genomic DNA library screening using the wild-type allele of the white gene as a probe. By comparing the mutant allele with the wild-type allele, the nature of the P factor might be deduced. As expected, mutations of the white gene induced by hybrid dysgenesis contained insertions, and the insertion sequences had all the characteristics of a transposable element. In fact, P factors were transposable elements and became known as P elements. Complete P elements were about 3 kb in length and contained four open reading frames encoding for a protein essential for P element movement. The terminal sequences of P elements consisted of inverted repeat sequences of 31 bp. In structure, the P elements generally resembled other transposable elements that had been isolated from bacteria and were referred to as short, inverted repeat-type transposable elements.
The physical isolation of an active transposable element from D. melanogaster provided researchers with a unique opportunity to integrate foreign DNA into the chromosomes of this species. Efforts to integrate exogenous DNA into the chromosomes of insects can be traced back to the late 1960s. While there was an interest in genetically transforming insects and a few reports of minor successes, there was no reliable method for creating transgenic D. melanogaster. The P element solved that problem. It was reasoned that if the terminal, noncoding sequences of the element, which serve as signal sequences directing the cutting and pasting of the element, were attached to any piece of DNA, that piece of DNA would acquire the mobility properties of a P element. Furthermore, if this altered transposable element could be introduced into a cell that was going to form gametes and the element jumped (i.e., transposed) onto one of the chromosomes of this cell, then the gametes arising from this cell would be transgenic and would give rise to transgenic progeny. This reasoning proved to be precisely correct. Under the appropriate conditions, genes to which the terminal noncoding sequences of a P element have been attached can readily integrate into the chromosomes of presumptive germ cells and lead to the efficient creation of transgenic insects (Fig. 1 ).
This relatively simple technology helped fuel a revolution in the study of this model organism. Today, this transposable element forms the basis for a suite of technologies that allow researchers to identify and analyze genes in a variety of ways. The P element gene transformation
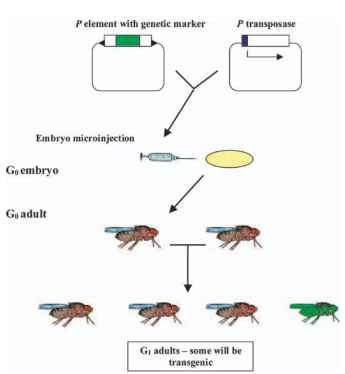
FIGURE 1 P element transformation of D. melanogaster. Two plasmids, one containing a P element into which has been cloned a genetic marker (green) and a helper plasmid containing the P element transposase (white) placed under the control of an induc-ible promoter (blue) are
system has also served as a paradigm for the development of similar tcoinjected into embryos. The P element inverted terminal repeats are shown as black arrowheads. G0 adults arising from injected embryos are not transgenic but some will contain a percentage of gametes containing the P transposable element. These adults are outcrossed and G1 progeny are examined for the presence of transgenic individuals (green fly).echnologies applicable to other species of insects.
GENE TAGGING WITH TRANSPOSABLE ELEMENTS
The key to the identification and isolation of P elements was the availability of the cloned white gene. The white locus was used as a trap; once the P element had been identified and cloned, it could be used as a way of identifying and isolating genes. As already discussed, one of the prominent features of P element movement (as revealed by the phenomenon of hybrid dysgenesis) was the creation of mutations. These mutations are caused by the insertion of the P element into an essential region of a gene, thereby altering its level or pattern of expression.
Mutations and their associated phenotypes define genetic loci. The existence of a mutant insect with an altered eye color defines a locus that plays some role in eye pigmentation. Although the existence of a mutant reveals the presence of a gene and its location, it does not provide researchers with a means of readily isolating the DNA containing the gene. If, however, the mutation is caused by the insertion of a sequence, such as a P element, and we know the sequence of the insertion sequence, we can use this information to isolate the DNA of the gene that was mutated. By making a genomic DNA library from the mutant insect, one can use conventional DNA hybridization techniques to identify sequences in the library that contain the P element. Because the mutation was caused by the insertion of the P element into a gene, the DNA adjacent to the P element is likely to be the gene responsible for the mutant pheno-type. This methodology of transposon tagging is very powerful and has been used not only in D. melanogaster but in a number of other organisms as well. The requirements for an effective transposon-tagging system that ensures unambiguous gene identification are an active transposon that has little integration site specificity and insect strains that contain few or only one transposon-tagging transposable element. Roberts has described the use of the P element for gene tagging and enhancer trapping.
ENHANCER TRAPPING
Transposable element-based muta-genesis or transposon tagging is a powerful technology with one limitation: it can identify only genes that have a recognizable mutant phenotype following element integration. Many of the genes that one mutates either do not result in a visible phenotype or cause the death of the organism. Such genes will never be recovered from a screen based on transposon tagging.
A complementary methodology that does not rely on mutagenesis for gene identification is called enhancer trapping. Enhancers are gene expression regulatory elements, and they function to fine-tune the control of gene expression, temporally and spatially. They are quite distinct from gene promoters in that enhancers are not sites of RNA polymerase binding but are instead sites for protein binding that influence when and how often RNA polymerase will associate with a promoter. A remarkable and useful feature of enhancers is their ability to act over long distances by mechanisms that are not entirely clear. That is, an enhancer may be located hundreds or even thousands of bases away from its target promoter. If a new promoter is inserted near the enhancer, it too will become regulated by that enhancer. This phenomenon provides a clever, nonmutagenic method for gene identification based on patterns of gene expression called enhancer trapping.
Like transposon tagging, enhancer trapping relies on the movement of a transposable element. The element in this case has been engineered to contain a gene whose expression is readily detected. Today the green fluorescent protein (GFP) from the jellyfish is a common choice. The reporter gene has been engineered to contain a minimal basal promoter, meaning that it contains an RNA polymer-ase binding site but no associated enhancers. Consequently, this enhancerless gene construct does not result in reporter gene expression unless the transposon in which it is contained integrates near an active enhancer. The presence of enhancers can be detected by moving the transposon around the genome and looking for expression of the reporter gene. By identifying enhancers with particular properties, one then has indirectly identified the genes controlled by these enhancers. Often, the genes regulated by enhancers identified by using this method are located in the proximity of the enhancer. The significant difference between this method of gene identification and transposon tagging is that enhancer detection does not require mutating the enhancer or its associated gene. Consequently, genes that may not have been detected by a transposon-tagging screen might be detected using an enhancer trap (Fig. 2 ).
Transposon tagging and enhancer trapping are rather intense genetic methods for gene identification. Such methods require the ability to efficiently perform genetic crosses, to recognize mutants or desirable reporter gene expression patterns, and then to maintain large numbers of distinct genetic lines of insects. Although Drosophila is readily amenable to such manipulations, other insects may be less so. Nevertheless, these methods will be of great value to those entomologists working on a variety of insect species.
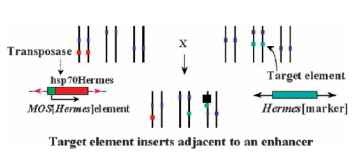
FIGURE 2 Example of enhancer trapping in insects: three pairs of chromosome with their centromeres (purple). One strain contains a MOS element into which the Hermes transposase has been cloned (orange). The MOS inverted terminal repeats are shown as pink arrowheads. A second strain contains a Hermes element containing a genetic marker (blue) placed under the control of a weak promoter. The two strains are crossed, whereupon the Hermes transposase causes the Hermes elements to move to new regions of the insect genome. Should a Hermes element insert near an enhancer element (black box), the genetic marker in the Hermes element would show the same tissue- and stage-specific expression of the gene controlled by the enhancer. The gene and the enhancer can then be cloned by standard gene tagging techniques.
HOMOLOGOUS RECOMBINATION
Transposon tagging and enhancer trapping are methods of identifying genes based on a phenotype: a mutant phenotype in transposon tagging, an expression phenotype in enhancer trapping. The availability of essentially the entire DNA sequence of the genome of D. melanogaster has permitted the identification of genes based entirely on DNA sequence patterns. Often the role of these genes is completely unknown because flies with mutations in these genes have not been identified. Without the ability to examine the phenotypes of flies with mutant alleles of the gene, gene function must be deduced entirely by other means, such as patterns of expression or analysis of the protein gene product. Today, however, it is possible for researchers who know the DNA sequence of a specific gene to create D. melanogaster with mutations in that gene. This method of targeted mutagenesis relies on the process of homologous recombination.
Homologous recombination, the process of gene exchange that typically occurs during meiosis, depends on the association of DNA sequences that are identical or nearly identical. Breaks in one of the strands of a DNA duplex can result in this strand becoming associated with its homolog on another chromosome, leading to gene exchange. It is now possible to exchange a gene located on a chromosome of a fly with a nearly identical gene created in the laboratory. This somewhat involved process relies on the use of a site-specific recombinase and a site-specific endonuclease, but it is potentially a method that will be generally applicable to any insect. Rong and Golic have described this technology in D. melanogaster.
The strategy behind using homologous recombination takes advantage of the high recombinogenicity of linear molecules of DNA. Such molecules will preferentially recombine with sequences homologous to the sequence at the end of the linear molecule. Gene targeting by homologous recombination in D. melanogaster is based on a clever method for generating the highly recombinogenic targeting molecule i n vivo. The process begins by creating a transgenic insect using, for example, a P element gene vector that contains the targeting sequences flanked by site-specific recombination sites such as the FRT sites of the FLP recombinase system. When FLP recom-binase is expressed (from a previously integrated transgene) in the insect, the FRT sites will recombine causing the targeting gene to be excised from the integrated gene vector. This recombination event results in the creation of extrachromosomal circular molecules in the nuclei of the insect. These extrachromosomal circles are then linearized by expressing a site-specific endonuclease (from a previously integrated transgene) that recognizes a DNA sequence that has been placed in the targeting gene in such a way that digestion results in the target gene sequences being located at the ends of the linearized circle. This highly recombinogenic molecule will then recombine with the chromosomal homolog, resulting in gene disruption.
Homologous gene replacement has been achieved for two Drosophila genes, the yellow gene and the pugilist gene, and most likely will be applicable to a large number of D. melanogaster genes. In particular it should enable gene function to be assigned to the thousands of new genes identified in the Drosophila genome project through replacing the wild-type forms with nonfunctional mutations that have been created in vitro.
A prerequisite for targeted gene replacement is a set of trans-genic insects that can express the appropriate restriction enzyme and the FLP recombinase. This is readily achieved in D. melanogaster and now can also be accomplished, in principle, in other insect species because transposable elements exist that can be used to genetically transform them. The FLP recombinase system has been shown to function correctly in the yellow fever mosquito, Aedes aegypti, and most likely will function in all insects into which it is placed. Similarly, the ability of a yeast restriction enzyme to function in Drosophila suggests that it should also function correctly in a range of insect species into which it is placed.
GENETIC ENGINEERING IN NONDROSOPHILID INSECTS
Genetic Transformation of Nondrosophilid Insects
The P element paradigm is successful in nondrosophilid insects. Despite many attempts, the P element was found to be unusable as a gene vector in nondrosophilid insect species. The reason for the narrow host range of P is unknown; however, it has been proposed that P is dependent for its mobility, in part, on the presence of host-encoded factors. These are thought to be absent, or at least sufficiently diverged, to prevent the mobility of P in these species. The P element is, however, not required for insect transformation because of the discovery and performance of four transposable elements, each from a separate family of transposable elements. Each of these is endowed with a broad host range, and each can transform D. melanogaster as well as a number of nondrosophilid species. They are briefly described below.
What is conserved between drosophilid and nondrosophilid transformation has been described as the P element paradigm. This refers to the mode of transformation. The P element and the four elements described shortly are class II transposable elements. They all transposase by a “DNA-only” type of mechanism—no production of an RNA intermediate is needed. These elements have an overall structure that is shared between them. They are short (<4kb), have inverted terminal repeated sequences, and encode a transposase enzyme that catalyzes the movement of the transposable element from one genomic location to the next. The same methodology is used to introduce these transposable elements regardless of species. Typically, two plasmids are coinjected into preblastoderm embryos. One plasmid contains the transposable element, into which has been placed a genetic marker and an effector gene—a gene meant to alter the phenotype of the insect in a desired way. The placement of the
marker gene and the effector gene interrupts and inactivates the transposase gene within the element, necessitating the use of a second plasmid containing the corresponding transposase, which is typically placed under the control of an inducible promoter such as the hsp70 promoter of D. melanogaster. This transposase mediates the transposition of the transposable element from the donor plasmid to the genome of the developing germline cells. As for D. melanogaster transformation, the individual arising from the injected embryo is not transformed; rather, it contains genetically transformed gametes. Individuals are mated, and transgenic insects are screened for in the next generation.
TRANSPOSABLE ELEMENTS USED FOR NONDRO-SOPHILID INSECT TRANSFORMATION
Four transpos-able elements can be used to genetically transform nondrosophilid insects: piggyBac, Hermes, Mariner, and Minos.
PiggyBac The 2.5-kb piggyBac element has 13-bp inverted terminal repeats and 4-bp direct repeats located proximally to these. It contains a 2.1-kb open reading frame that encodes a transposase enzyme. piggyBac was discovered through its ability to transpose from the chromosomes of the Cabbage looper Trichoplusia ni into the genome of a baculovirus that had infected this TN368 cell line. Transposition of piggyBac into the baculovirus genome led to a mutation that resulted in few polyhedra being generated, in turn causing a clear change in cell morphology. piggyBac inserts only at TTAA sites and generates duplications of this sequence at the target site. Excision of piggyBac is precise—unlike other class II insect transposable elements, no deletions or additions of DNA remain at the empty excision site. piggyBac has found wide use as a gene vector in insects and has been used to genetically transform the flies C. capitata, Bactrocera dorsalis, Anastrepha suspensa, Musca domes-tica, L. cuprina, and D. melanogaster; the mosquitoes Anopheles albimanus, Anopheles stephensi, Anopheles gambiae, and Anopheles aegypti; the moths Bombyx mori and Pectinophora gossypiella; and the beetle Tribolium castaneum. Little is known about the distribution of piggyBac throughout insects, although highly similar elements have recently been found in three strains of B. dorsalis. Over the 1.5 kb of nucleic acid sequence examined, these B. dorsalis elements are 95-98% identical to the element originally isolated from T. ni cells. Two of these B. dorsalis piggyBac-like sequences contain small deletions that interrupt the open reading frame, whereas the third has an intact open reading frame over the region examined. Conceptual translation of this region yields a sequence identity of 92% compared with the corresponding region of the T. ni piggyBac tranposase. The basis of the distribution of piggyBac-like elements combined with the possible effect that incumbent piggyBac-like sequences may have on introduced elements in transgenic lines is a fertile field for investigation.
Hermes Hermes elements are members of the hAT family of transposable elements that are widely dispersed in animals and plants. Some members of this family, such as the Ac element of maize and the Tam3 element of snapdragon, have a broad host range, and this attribute is shared with the Hermes element. Hermes was isolated from the house fly, M. domestica, and was first recognized by its ability to cross-mobilize the related hobo element when this was introduced into house fly embryos by microinjection. The 2.7-kb Hermes elements contain 17-bp inverted terminal repeats and a 1.8-kb open reading frame that encodes a transposase of 70 kDa. Hermes elements exhibit a preference for inserting at 5′-GTnnnnAC 3′ sites and create 8-bp duplications of these sites upon insertion. They have been used to genetically transform D. melanogaster, C. capitata, Stomoxys calcitrans, A. aegypti, Culex quinquefasciatus, and T. cas-teneum. Plasmid-based transposition assays have shown that Hermes can transposase in several other insect species as well. Hermes transposes by a cut-and-paste mode of transposition in higher Diptera but seems to integrate by another, transposase-dependent mode in mosquito germlines. The molecular basis of this remains unknown. Hermes elements can interact with the related hobo element (and vice versa) when both are present in the genome of D. melanogaster.
Mariner Mariner elements are widespread among arthropods. They are approximately 1.3 kb with inverted terminal repeats typically around 30 bp long. Mariner elements can be present in an extremely high copy number in some species; however, it seems likely that only a handful (if any) of these may contain a single open reading frame that encodes an active transposase of approximately 33 kDa. Based on DNA sequence comparisons, five different subfamilies of Mariner elements exist in arthropods. The distribution of members of these subfamilies is inconsistent with the established evolutionary histories of their host species and it is now accepted that Mariner elements have been horizontally transferred throughout evolutionary time. At present only one naturally occurring, active Mariner element has been discovered. This is the MOS element from Drosophila mauri-tiana and has been used to genetically transform D. melanogaster, A. aegypti, and M. domestica. Indeed MOS displays a broad host range and has been used to genetically transform Leishmania, chickens, and zebrafish. The mobility characteristics of MOS are preserved in these species; it transposes by a cut-and-paste mechanism and inserts at, and duplicates, TA nucleotides. A second active element, Himar, was constructed based on a consensus of Mariner sequences obtained from the horn fly, Haematobia irritans. Himar is active in Escherichia coli but so far is inactive in insects.
Minos The Minos element is a member of the Tc 1 family of trans-posable elements. The Tc1 family of elements is related in sequence and mobility properties to the Mariner family of elements, and both are grouped into a single superfamily of elements. Minos elements are approximately 1.8 kb and possess long, 254-bp inverted terminal repeats. Minos contains two long open reading frames that are interrupted by an intron. Conceptual translation of the Minos transposase gene reveals a greater than 40% identity with the Tc1 transposase of Caenorhabditis elegans. Minos has been used to genetically transform C. capitata, D. melanogaster, and A. stephensi.
Transposable Elements in New Hosts
These four transposable elements just discussed provide the means by which genes can be introduced into pest insect species. Although these elements represent four different transposable element families, the transformation frequencies achieved, with some exceptions, are in the range of 1-10%. It seems likely that all will enjoy use as gene vectors in a range of insect species, and all may well be subject to interactions with endogenous transposable elements or other host factors present in these species. This is an important point that is not encountered by geneticists working on Drosophila. The recipient strains used for P element transformation are devoid of P elements (and any other related elements) and are deliberately chosen for this reason. This is not possible in other insect species in which the composition of the target genome with respect to transposable elements is unknown.
Whether interactions with endogenous transposable elements and/or host factors occur at levels that detrimentally affect transgenic stability is an issue that must be addressed. Central to this is development of a complete understanding of how these transposable elements are regulated both in their original host species and in species into which they have been introduced.
Genetic Markers
The development of universal genetic marker genes, together with the identification of promoters to drive their expression in het-erologous species, has played a major role in the extension of genetic engineering into nondrosophilid insects. Natural and modified forms of the GFP gene of the jellyfish, Aequoria victoria, have enabled transgenic insects in several species to be easily identified from non-transgenic siblings at most stages of development. These include D. melanogaster, C. capitata, B. dorsalis, A. aegypti, A. stephensi, C. quinquefasciatus, P. gossypiella, T. casteneum, and S. calcitrans. In these species, the GFP gene has been placed under the control of a promoter that enables either organelle-specific or tissue-specific expression of the marker gene to occur. Examples of the former are the actin5C and polyubiquitin promoters of D. melanogaster. Examples of the latter are the Pax6 and actin88 promoters. The actin88 promoter is from D. melanogaster and is specifically expressed in the indirect flight muscles of the pharate adult and adults. Pax6 is a member of the Pax family of transcription factors and is specifically involved in the development of the eye and central nervous system.
The enhanced GFP (EGFP), cyan fluorescent protein (CFP), yellow fluorescent protein (YFP), and Ds Red forms of the fluorescent protein genes can also function as genetic markers in insects.
OTHER APPROACHES TO GENETIC ENGINEERING IN INSECTS FLP/FRT
Recombinase in Nondrosophilid Insects
The FLP/FRT recombinase system of the yeast Saccharomyces cerevisiae can also function correctly in at least one nondrosophilid species. Plasmid-based excision and integration assays showed that the FLP recombinase enzymes could recognize and recombine FRT sites in the soma of developing A. aegypti embryos. Excision at the FRT sites was high—60% of plasmids examined had undergone an excision event mediated by FLP recombinase. Integration, as measured by the formation of heterodimeric plasmids arising from the recombination between two plasmids each containing an FRT site, occurred at a low, but statistically significant, frequency. The ability of the FLP/FRT recombinase system to function correctly in Drosophila and Aedes suggests that it should function across a range of insect species. It cannot, however, be used to directly genetically transform an insect species because to achieve this, FRT sites must first be introduced into the target genome by some other means, such as transposable elements. When combined with transposable element technology, this yeast recombination system should allow investigators to undertake precise manipulations of both introduced and host DNA. This ability will be of particular importance if DNA sequences necessary for the movement of transposable elements need to inactivated (e.g., for regulatory reasons) following initial integration of the element into the target genome.
RNA-Mediated Interference (RNAi) in Insects
RNA-mediated interference (RNAi) refers to the targeted disruption of gene expression arising from the introduction of double-stranded RNA (dsRNA) into the cell. This disruption is targeted only to RNA molecules homologous to the invading dsRNA. It was initially characterized in plants and in the nematode C. elegans but is now thought to be a general phenomenon of eukaryotic cells that enables them to overcome invasions of RNA viruses. The mechanism by which RNAi works is unknown. It does not work through a simple titration of nascent or messenger RNA as would occur for an antisense RNA-based mechanism because the RNAi response can be elicited by far fewer dsRNA molecules per cell than, target RNA molecules. A catalytic mechanism in which the presence of dsRNA induces the destruction of homologous cellular RNAs has been recently proposed. RNAi technology has been harnessed to allow the targeted inactivation of specific genes and will prove to be a valuable component of genomics projects in those species in which nucleic acids can be introduced into cells. In its original experimental design, the effects of RNAi were not inherited. RNAi technology has recently been combined with P transposable element technology in D. melanogaster to produce heritable RNAi-mediated gene inactiva-tion. Thus it is possible to examine the function of genes expressed in later stages of development of this insect and also the generation of genetically stable mutant lines in which production of the dsRNA can be induced or terminated based on the promoter used to drive expression of the extended hairpin loop RNA. RNAi technology should be extendable into other insect species in which transformation systems exist, and its exploitation in insects such as mosquitoes will enable the effects of the selective inactivation of specific genes to be quickly determined. This will represent a significant advance over traditional methods of creating and isolating mutants in these insect species that have not traditionally been amenable to genetic analyses.
EXAMPLES OF INSECT GENETIC ENGINEERING FOR INSECT POPULATION CONTROL
Transgenic technology in nondrosophilid insects has already been applied to examine promoter function and gene expression in trans-genic lines of A. aegypti and C. capitata. In addition, recent work performed in D. melanogaster illustrates how transgenic approaches may be applied to pest insect control in the foreseeable future. This approach involves using transgenic technology to develop new genetic sexing strains. Although these experiments have been performed in D. melanogaster, the concepts are applicable to any insect species in which transgenic technology has been developed, and the ability to generate and test novel genetic strains in pest insect species should result from such additional experiments.
Both systems were centered on exploiting the tetracycline-controlled transactivator (rTA) gene, which is inactivated in the presence of tetracycline. As a dietary component, tetracycline can readily be administered to Drosophila larvae in measured doses. Both systems consist of two components, which are combined in a single strain when transgenic lines containing each component are crossed. The rTA gene was placed under the control of the enhancer from the yolk protein 1 (ypl) gene of D. melanogaster. This enhancer results in fat-body- and female-specific expression of the yp1 gene. The second component of their system was a proapoptosis gene (head involution defective—hid), the expression of which leads to apoptosis and the death of the organism. The hid gene was placed under the control of the tetracycline operator (tetO), which contains the binding site for the rTA protein. Thus, in females the ypl-rTA gene is induced and, in the absence of tetracycline in the diet, the rTA protein binds to the tetO sequence and so induces the expression of the hid gene. All transgenic females that were raised in the absence of tetracycline and possessed both components of this lethal genetic system died. When tetracycline was added to the diet, the rTA protein was inactivated, and there was no female lethality. Males containing both components were unaffected on either diet.
These experiments clearly demonstrate that transgenic technology can be used to construct efficient genetic sexing strains in at least one species of insect—D. melanogaster. The genes, promoters, and enhancers chosen to do so are predicted to be of generic use in insects. The tetracycline-controlled transactivator system is from bacteria and, given that it functions correctly in Drosophila, will most likely be applicable to all insects in which tetracycline, or its analogs, can be delivered in measured doses. Female-specific enhancers would be expected to exist in nondrosophilids, should the D. melanogaster enhancers not function correctly in these species. Similarly, should conditional lethal alleles of Drosophila genes not function in other species, it should be possible to generate analogous mutants either by established procedures or by employing an RNAi-based approach. The extension of these strategies into nondrosophi-lid insects requires, in the end, genetic transformation procedures and, as already discussed, several of these now exist for nondrosophi-lid insect species.
CONCLUDING REMARKS
For many years, the absence of genetic transformation techniques for nondrosophilid insect species was seen as a bottleneck for the full extension into these important pest species of strategies based on molecular genetics. The development of successful transposable-element-based transformation technologies enables the potential of these strategies to be tested at last. Insect geneticists have at their disposal gene vectors, universal genetic markers, promoters that can be utilized in heterologous insect species, and many target genes to test and manipulate. In addition, as outlined here, there is reason to be confident that generic techniques such as gene tagging, enhancer trapping, homologous recombination, FRT/FLP recombination, and RNAi-based gene silencing can now also be applied to insects other than D. melanogaster. Reports of sex-specific lethal genetic systems working in Drosophila have been published, and there is every expectation that similar systems will soon be established and tested in pest insects. All these technologies are precise—targeting only the genes that investigators seek to change—and the effects on a laboratory population can be predicted and are unambiguous. How successfully these technologies can be extended into pest insects, both in the laboratory and in the field, will be a matter of some interest in the years ahead.
