Dichloro-diphenyl-trichloroethane (DDT) is an old insecticide that has been banned from use in most countries of the world since the 1970s. However, DDT, its metabolites, and some of its derivatives, which are mostly produced as impurities in technical insecticide preparations, still contaminate the environment. DDT residues continue to cause deleterious biological effects, most notably, environmental endocrine disruptions.
From the viewpoint of environmental toxicology and chemistry, DDT is by far the best-studied chemical. Many models of bioaccu-mulation, atmospheric transport, transfer mechanisms within soil compartments, and from soil to air, and soil to water are based on data generated from studies of DDT residues in the environment.
CHEMICAL CHARACTERISTICS
DDT is one of several typical chlorinated hydrocarbon insecticides discovered in the early 1940s and known for their persistent insec-ticidal activities, their lipophilic attributes, and their stable chemical properties. The insecticidal properties of DDT itself were discovered in 1939 by Paul H. MiiHer of Switzerland, who later received the Nobel Prize for his work. Since DDT was the first organic synthetic insecticide that possessed advantages such as low mammalian toxic-ity, wide spectrum, long-lasting properties, and low cost in comparison to arsenicals and other inorganic insecticides, most entomologists embraced its use to such an extent that more than 100 million pounds of DDT was being produced annually by the mid-1950s.
The insecticidal active ingredient of DDT preparations is p,p’-DDT (Fig. 1A). Its 1-dechlorination product, p,p’-DDD (Fig. 1B), retains reasonable levels of toxicity for some insects, but its dehy-drochlorination product, p,p’-DDE (Fig. 1C), shows no insecticidal property, although p,p’-DDE could still have a toxic effect in other organisms. Other components often found in insecticidal DDT preparations are o,p’-DDT, p,p’-DDD, and o,p’-DDD. All these can be found as environmental residues.
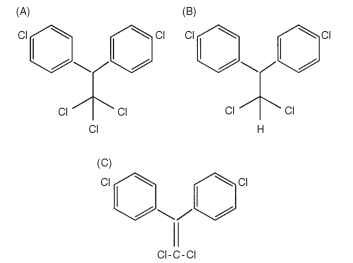
FIGURE 1 (A) 1,1,1-Trichlor-2,2-bis (p-chlorophenyl) ethane
p.p’ DDT ; (B) p,p’-DDD; (C) p,p’-DDE.
Dichloro-diphenyl-ethylene (DDE), one of the residues derived from DDT most frequently found in the environment, is produced mainly by metabolic activities in biological systems and is particularly prevalent in insects and in some mammalian species. Although both p.p’-DDE and o.p’-DDE are found in the environment, the former is more abundant and more frequently encountered. In assessing residue levels of all DDT-derived compounds today, scientists express the entire spectrum of DDT-related (DDT-R) compounds or DDT-derived compounds as total DDT residue, or DDTs.
EFFECTS ON INSECTS
The main action mechanism by which p,p’-DDT causes the death of insects is the destabilization of the sodium channel, the main vehicle that propagates excitation signals on the surface of neurons, so that affected neurons become easily excitable. Insects poisoned by DDT show typical hyperexcitation symptoms that lead to exhaustion and death. This phenomenon may be better understood as an elec-trophysiological manifestation in which neurons affected by DDT show a typical excitation pattern called “repetitive discharges.” Such a neuron that has been excited by a stimulus remains in an excited state and continues to discharge for several minutes.
The most well-known use of insecticidal DDT is probably for mosquito control in malaria-eradication programs. The most frequently used technique was that of “wall painting” of the interior of buildings with DDT in areas where malaria was prevalent. Because mosquitoes transmit malaria directly from human to human (i.e., without going through other hosts), this method effectively cuts off the link to continued transmission. The two key properties of DDT responsible for its effectiveness are the extreme susceptibility of mosquitoes to DDT and the long-lasting nature of DDT, particularly in indoors and dry environments.
DDT was also well known for its role in the control of cotton insect pests that posed a serious problem to cotton growers in the southern United States. The most commonly used formulation was a mixture of DDT and toxaphene. DDT was also used to control many other pests including the bark beetle vectoring Dutch elm disease, locusts, and forest pests (e.g., spruce budworm); these wider uses resulted in environmental loading of DDT-R.
ENVIRONMENTAL EFFECTS
Although p,p’ -DDTis really the only component of DDT-R potent enough to be an insecticidal ingredient (as far as environmental effects are concerned), all the DDT-related compounds are presumed to be potentially toxic. Perhaps the best example of the extreme toxicity of DDE is its effects on bird reproduction. Because DDT is slowly converted into DDE in the environment over many years, environmental samples of DDT-R today are actually mostly DDE.
Another important compound is o,p’-DDT, which is known to mimic the actions of estrogen in several vertebrate biological systems. The action of o,p’ -DDT can be attributed to its ability to bind to the estrogen receptor as an agonist, like estrogen itself, and to activate estrogen signals in the organism. Interestingly, p,p’-DDE acts as an antagonist to the androgen receptor in males, thereby blocking male sex hormone signaling in many vertebrate species.
Of all the effects of DDT-R compounds on wildlife, the biological damage cited most frequently is that of eggshell thinning. This phenomenon was originally reported by Ratcliffe in 1967 and verified by Anderson and Hickey in 1976 in North America. In addition to DDT, both DDE and polychlorinated biphenyls also have deleterious effects on eggshell production. Eggs affected by these chemicals crack easily and contribute to the decline of vulnerable bird species.
Eggshell thinning is not the only harmful effect for which DDT-R has been implicated. DDT-R has also been shown to contribute to the increased mortality as well as myriad reproductive problems among a broad range of wildlife including birds, fish, and other aquatic organisms. Behavioral changes are also caused by exposure to DDT-R.
A current view among scientists is to interpret many of these effects as ” endocrine disruptions” caused by the hydrocarbon pollutants, with DDT-R being one of the prominent study materials. Certainly, DDT-R, particularly o,p’-DDT, acts in an estrogen-like manner, whereas p,p’-DDE acts as an anti-androgen. Deleterious effects of such endocrine disruptions by DDT-R in birds are well documented. Because disruptions of endocrine actions, including those of some vitamins, are expected to cause serious effects on reproduction,development, and nutritional balance of animals, this topic is likely to attract increased attention in the scientific community.
Despite the difficulty of conducting and evaluating environmental effects studies, evidence for the harmful biological effects of DDT on wildlife and ecosystems has been overwhelming. Clearly, the decision to ban the use of DDT was sound.
