Chromosomes in insects display almost the whole range of var-■ iation seen in the chromosomes of higher plants and animals.
In these groups the deoxyribonucleic acid (DNA), which contains the genetic code determining development and inheritance, is contained in a nucleus in each cell. At interphase, the DNA is organized into the complex linear structures that are chromosomes, which can be seen in a conveniently condensed state when the cell is dividing.
The study of insect chromosomes is less intensive now than formerly for three possible reasons: (1) the thoroughness of the early investigators, (2) the commercialization of science, which has pushed the study of chromosomes (cytogenetics) in animals toward more lucrative mammalian, and particularly human, fields, and (3) the replacement of cytogenetic with molecular methods. The third point was predicted by Michael White in the conclusion to his famous 1973 text topic, Animal Cytology and Evolution. Through his work, almost entirely on insects, White is widely regarded as the founder of the study of evolutionary cytogenetics in animals and one of its foremost authorities; his topic remains a most comprehensive authority on most aspects of insect chromosomes.
In 1978 White was firmly convinced that evolution can be a cytogenetic process, and he did much to demonstrate this at the level of spe-ciation. At a higher evolutionary level, the integrated chromosomal characteristics of the various insect orders seem to support this view. However more recently authors such as King have de-emphasized the evolutionary importance of chromosomal changes in evolution.
SOURCES AND PREPARATION OF CHROMOSOMES FROM INSECTS
Mitotic chromosomes undergoing the stages of prophase, met-aphase, anaphase, and telophase can be prepared from any insect somatic tissues with dividing cells. Embryos are the best sources of mitotic divisions, but they are also seen in the midgut ceca of adults and juveniles and in the follicle cells covering very early ova in females.
Insect cytogeneticists now usually use colchicine or other mito-static agents to arrest the chromosomes at metaphase of mitosis by inhibiting the formation of the spindle fibers required for the cells to progress to anaphase. Squashing, under a coverslip, spreads the chromosomes, and for squash preparations the cells are usually prestained. Insect cytogeneticists now often use air-drying to spread the chromosomes, since this process has the advantage of making the chromosomes immediately available for modern banding and molecular cytogenetic methods.
Male meiosis is very commonly used to study the chromosomes of insects and to analyze sex-determining mechanisms. The structure of the insect testis is very favorable to chromosomal studies because each lobe has a single apical cell that divides by a number (s) of spermatogonia! divisions (Fig. 1A) to yield 2s primary spermatocytes, which then undergo synchronous first and second meiotic divisions to yield 2s+1 secondary spermatocytes and 2s+2 sperm.
First meiotic prophase in insects involves the usual stages (Fig. 1). Replication of the DNA is followed by the prophase stages of leptotene
FIGURE 1 Mitotic and meiotic holocentric chromosomes in an earwig, Labidura truncata. Orcein-stained squash preparations, B, L, M-P colchicine-treated. (A) Spermatogonial division in prophase with the Y at bottom left and the X to the right, both more condensed than the autosomes. (B) Spermatogonial metaphase with the small Y chromosome obvious. (C) Leptotene, with the sex chromosomes at the top very condensed and the heterochromatic ends of some autosomes also condensed. Two nucleoli are visible, one at 11 o’clock and the other at 5 o’clock. (D) Zygotene-pachytene with the heterochromatic ends of the autosomes more obvious. (E) Diplotene displaying the four autosomal bivalents and the condensed sex chromosomes separately. (F) Diakinesis, one autosomal bivalent showing a chiasmata that is quite interstitial. (G, H) First metaphases with the larger X seem to be paired with the smaller Y. First anaphase with the neocentromere actively moving the chromosomes apart. (J, K) Second metaphases; J shows the X dyad, K shows the smaller Y dyad. (L-P) Female mitotic chromosomes, late and early prophase in L and N, respectively; M-P show metaphases, with O and P showing secondary constrictions. The primary constrictions of fixed centromeres do not show, and uninterrupted chromatids, characteristic of holocentric chromosomes, are particularly obvious in M.
(strand forming), zygotene (chromosome pairing to form bivalents), pachytene (crossing over to yield recombinants), diplotene (repulsion of the homologues), diakinesis (completion of repulsion), and premet-aphase (bivalents fully condensed).
Metaphase I is followed by first anaphase, which can be a very informative stage and, in contrast to mammals, is readily available in insects. Second meiotic division is also readily observed in insects (Fig. 1) and can be useful for confirming events in earlier stages.
Meiotic chromosomes in insect females are difficult to prepare and are usually studied only in special cases, such as parthenogenesis.
TYPES OF CHROMOSOME IN INSECTS
Autosomal chromosomes are usually represented as two haploid sets, one from each parent, in the diploid tissues of insects. With the addition of the sex chromosomes from each parent, the haploid set is known as “n” and the diploid set as “2n. ” Major exceptions to diploidy in both sexes occur, such as in almost all species in the orders Hymenoptera (ants, bees, and wasps), Thysanoptera (thrips), and some species of Heteroptera and Coleoptera, where the females are diploid, the males being normally haploid (i.e., derived from unfertilized eggs, arrhenotoky). Arrhenotoky determines the sex of about 20% of all animal species. This mechanism has allowed one species of Australian ant, Myrmecia croslandi. to achieve the lowest possible chromosome number, n = 1, in the parthenogenically derived male.
Chromosomal imprinting has not been demonstrated in insects, so gametes from both sexes are not necessarily required. Indeed, accidental development of unfertilized eggs (thelytoky) can form a parthenogenetic insect if sufficient double-haploid cells arise in critical tissues in the n/2n mosaic.
Sex chromosomes are usually involved in sex determination in insects, but by a variety of genetic mechanisms. The male is usually the heterogametic sex in insects, the exceptions being the orders Lepidoptera (butterflies and moths) and Trichoptera, in which the females are heterogametic. The mammalian system of having genes determining the sex and other male functions on the Y chromosome almost certainly does not occur in insects. In the earwigs (Dermaptera), male determination by the presence of a Y chromosome seemed to be the rule, until XO/XX mechanisms were found in two species.
As in other animals, the insect heterogametic male has half the number of X chromosomes as the female; most commonly the sexes are XO male and XX female, but multiple X-chromosome systems frequently occur. Fusions of autosomes to the X chromosome can cause the formation of XY/XX and further fusions to form X1 X2Y/ X1X1X2X2 systems. X1X2Y males are almost the rule in the mantids (Mantodea). In the most of the Hymenoptera, sex is determined by the diploid females being heterozygous, and the haploid males hemizygous, for multiple alleles at a single genetic locus on one chromosome; that might still be regarded as an X chromosome.
The karyotype is the set of chromosomes, both autosomes and sex chromosomes, in an organism. The karyotype found in 90% of the large family of short-horned grasshoppers, Acrididae, is usually given as 2n d- = 23 [22 + X (or XO)], the female karyotype, 23 (22 + XX), being usually inferred from the male. Some authors carefully confirm diploidy and subdifferentiate the autosomes and the sex chromosomes, for example, for the earwigs Chaetospania brunneri 2n c? = 31 (13AA + X1X2X3X4Y) and Nala lividipes. with one pair of autosomes being exceptionally long, 2n d1 = 34 (ALAL + 15AA + XY). By comparison, in mammals only the diploid count and the sex chromosomes are shown (e.g., human 2n d” = 46,XY).
Monocentric chromosomes are the norm in most insect orders (Fig. 2), with the single centromere characterized by a primary constriction, a structure seen in many other animals and in plants. After replication of the DNA and other chromosomal constituents during interphase, the chromosomes at metaphase show two identical chro-matids. Following the discovery that each chromatid must be terminated by a telomere, geneticists concluded that it is highly probable that a chromosome must always have two arms, one on each side of the centromere. If these arms are of appreciable length, the chromosomes are called metacentric (arms of about equal length), or submetacentric (arms of unequal length). If one of the arms is very
FIGURE 2 Monocentric chromosomes of the locust Chortoicetes terminifera, mostly acrocentric with some of the smaller ones sub-metacentric. (A) With one B chromosome, which is distinctively G-banded by a trypsin treatment that has produced comparatively minor effects in the A chromosomes. (B) With two B chromosomes showing positive C-banding for most of their length. The A chromosomes mostly have small centromeric C bands, but they show variable interstitial and distal C-banded segments.
short, perhaps invisible under normal microscopy, the chromosome is said to be acrocentric. It is now widely accepted that a chromosome cannot normally be telocentric (terminated by a centromere). The sequence of nucleotides repeated many times to make up the DNA of the telomeres of most insects is TTAGG, but it is not universal.
Holocentric chromosomes occur in the insect orders Heteroptera, Dermaptera (Fig. 1), Mallophaga, Anoplura, and Lepidoptera. The centromeres are elongated across much of the length of the chromosomes, although not usually extending to the telomeres. During mitotic anaphase, the spindle fibers pull equally on most of the length of the chromosome so that only the distal ends can be seen to be trailing. Operation of an elongate centromere during first meiosis would break the crossovers, or chiasmata, which have formed between the paired chromosomes. Apparently to preserve this chromosomal bivalent, the holocentric chromosomes develop neocentric activity at one telomeric end only. The neocentromeres behave like those of monocentric chromosomes, and they persist through second metaphase of meiosis (Fig. 1). Broken holocentric chromosomes seem to be able to retain attachment to the spindle fibers: each piece of a broken chromosome forms a bivalent with a neocentromere during meiosis. Breakage of holocen-tric chromosomes probably also explains the wide range of chromosome numbers seen in butterflies, from 2n = 14 to 2n = 446, and the extreme of 2n = 4 to 2n = 192 found by Cook in a single genus of scale insect, Apiomorpha . Breakage probably also accounts for the common finding of multiple X chromosomes in insects with holocentric chromosomes.
The addition of telomeres to the broken ends of holocentric chromosomes might be a function of the complex enzyme telomerase.
Polytene chromosomes are large chromosomes formed by the repeated replication, without intervening division, of chromatids that remain uncondensed as in interphase (Fig. 3 ). Polytene chromosomes often contain thousands of chromatid strands, and the homologous chromosomes are usually closely somatically paired, so that inversions in them are accommodated by the formation of loops. Transcription of ribonucleic acid (RNA) from the DNA is accomplished at expanded regions called Balbiani rings, (BR in Fig. 3B), and the attachments of the polytene chromosomes to the nucleoli (N in Fig. 3B) by the nucle-olar organizing regions are obvious. Polytene chromosomes have been most famously studied in the salivary glands and other glandular tissues in insects of the order Diptera, particularly in the fruit fly, Drosophila melanogaster. They display a large number of bands without any special staining, and the detail revealed is most useful for the localization of DNA sequences of various types, including single gene probes.
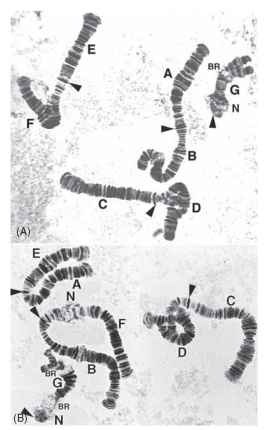
FIGURE 3 Polytene chromosomes in the salivary glands of the larvae of two species of chironomid midge. Orcein-stained squash preparations. (A) From the North American species Chironomus decorus, species b; (B) From the Australian species C. oppositus. For both species, labels A-F indicate arms of metacentric chromosomes, with arrowheads indicating the centromeres. The acrocentric chromosome G shows some breakdown of somatic pairing at the distal end in both species. Chromosomes AB and EF in C. decorus b have undergone whole-arm exchanges to form AE and BF chromosomes in C. opposi-tus. N and BR indicate nucleoli and Balbiani rings, respectively. Loop pairing, resulting from heterozygosity for paracentric inversions, can be seen in arms D and F in C. decorus b and in arm D in C. oppositus (Images kindly supplied by Dr. Jon Martin, University of Melbourne).
Supernumerary or B chromosomes occur occasionally in insects of most orders. B chromosomes, when present, are in addition to the always-present A chromosomes. Certainly the most variable and spectacular B chromosomes ever seen were found in the Australian plague locust, Chortoicetes terminifera (Fig. 2). These B chromosomes display over 20 different banding patterns after treatment with trypsin, and this treatment allowed the harmless identification of carriers of B chromosomes using interphase cells in the hemolymph, thus facilitating breeding experiments. These breeding experiments showed that single B chromosomes in males of C. ter-minifera were distributed into the sperm with a 50% frequency, but in females single B chromosomes were driven into the egg with a frequency of 80%. This meiotic drive in females should have ensured that every individual in the population carried a B chromosome. Since, however, they were found in only 10% of individuals, the B chromosomes must have been lowering the fitness of carriers. The situation supported a “parasitic” mechanism for the maintenance of B chromosomes in the population.
SUBCHROMOSOMAL ORGANIZATION IN INSECTS
Euchromatin and heterochromatin can be distinguished in insects in various ways. Euchromatin contains the active genes, and heterochromatin contains mainly repetitious, transcriptionally inactive DNA. Heterochromatic segments of the chromosomes can be observed in meiosis because of their high degree of condensation during first prophase (Fig. 1) . Heterochromatin may also be detected by hybridization in situ of repetitous DNA sequences, such as satellite DNA, to the chromosomes. The DNA of heterochroma-tin is replicated later in the S phase of the cell cycle than the DNA of the euchromatin. Examples of DNA replication that is both late to start and late to finish has been seen in the B chromosomes of C. terminifera, and in the sex chromosomes of the common earwig, Forficula auricularia. The C-banding technique can also be used to stain heterochromatic segments.
In most cases the heterochromatin of insects is constitutive (i.e., in a permanent state), but in some insects with peculiar life cycles, such as the Cecidomyiidae, individual chromosomes or sets of them may be made facultatively heterochromatic before being eliminated from the soma or the germ line of one of the sexes.
Chromosome banding in insects is largely limited to C-banding (Fig. 2B), originally named because the repetitious DNA proximal to the centromeres was stained. The position of the C bands is often procentric in insects, but these bands can be procentric, terminal, or interstitially distributed on the chromosome arms in different races of the same species, as in the grasshopper Caledia captiva.
The results of treating insect chromosomes with trypsin or other reagents that induce the narrow G bands, which are distributed all over the chromosomes of vertebrates, are disappointing in insects. The bands revealed by trypsin treatment of the B chromosomes of C. terminifera seem to be exceptional, and they are a reflection of the C-banding patterns of these chromosomes (Fig. 2 ).
POLYPLOIDY
Polyploidy occurs when the zygote, or first cell, has more than two sets of haploid chromosomes. In insects, polyploidy is mainly restricted to parthenogenetic species and is largely limited to 3n and 4n. Chromosomal sex determination is regarded as a major barrier to the formation of polyploids among bisexual species of insects because duplicated sex chromosomes, such as XXYY, would lead to uniformly XY sperm and therefore no possibility of sex determination.
Endopolyploidy is the occurrence of a multiplicity of the ploidy in the zygote in the somatic tissues of an organism. The term “endomitosis” is used if the chromosomes appear during cycles of endoredupli-cation but with no formation of a mitotic spindle and no cell division. Endopolyploidy is commonly seen in the somatic tissues of most insects. The formation of polytene chromosomes (Fig. 3) is also a special form of endoreduplication. Endopolyploid cells are very common in the tissues of all insects, and the phenomenon seems to reflect a tendency for insects to increase the bulk of certain tissues by increasing cellular size rather than cell number.
CHROMOSOMAL REARRANGEMENTS
Rearrangements occur in the chromosomes of insects when they occasionally break and rejoin in an irregular fashion. If any chromosomal rearrangement is maintained heterozygously in a population at a frequency greater than can be explained by recurrent chromosomal mutation, it is said to be polymorphic. There are a number of chromosomal rearrangements.
Paracentric Inversions
Paracentric inversions result when two breaks in one chromosome arm rejoin after the excised piece has inverted. These rearrangements are commonly recorded in polytene chromosomes, where the presence of them is shown by the formation of a loop allowing the homologues to be closely paired (Fig. 3). The presence of a chias-mata at meiosis within paracentrically inverted segments results in a dicentric chromosome and an acentric fragment, which cannot be regularly transmitted. Paracentric inversions survive for long periods in many dipteran species because there is no chiasma formation in males and because the products of female meiosis are organized to ensure that a nonrecombinant for any paracentric inversion is deposited in the egg nucleus.
Because they have the capacity to lock up long combinations of syntenic genes, it has been assumed that inversion polymorphisms can be adaptive. For paracentric inversions, many studies with dipter-ans have been undertaken to link paracentric inversion polymorphism to aspects of the environment in which the particular insect exists.
Pericentric Inversions
Pericentric inversions result from breaks in each arm of a chromosome that rejoin after the excised piece containing the centromere has inverted. Pericentric inversion polymorphism was perhaps most famously studied in the morabine grasshopper, Keyacris scurra. White and coworkers used this rearrangement to develop adaptive topographies (defined by Sewell Wright) for various populations of K. scurra that were on a saddle between adaptive peaks. The duplications and deletions that are the consequences of recombination within mutually pericentrically inverted segments seem to be largely avoided in insects bearing them at polymorphic frequencies.
Translocations
Translocations result from breaks in two chromosomes that allow exchange of pieces between the chromosomes. For breaks that are interstitial on the chromosome arms, a reciprocal translocation results, and in heterozygotes the translocated chromosomes synapse together at
meiosis to form a quadrivalent (or a multivalent, if exchanges are more frequent). Multiple translocation heterozygosity has been observed, with resulting ring multivalents, in the cockroaches (Blattodea).
Centric Fusions
Centric fusions, or Robertsonian translocations, are special cases of translocation in which two breaks are very close to the centromeres of acrocentric chromosomes, causing the formation of a large metacentric or submetacentric chromosome. Centric fusions commonly distinguish chromosomal races or species in insects, but they are not seen to be maintained polymorphic in populations as frequently as they are in mammals. Centric fusions between sex chromosomes and autosomes results in the formation of neo-XY and X1X2Y systems in insects.
Dissociation
Dissociation, the reverse of fusion, involves the formation of two acrocentrics from a metacentric chromosome. Dissociation is rare because a donor centromere, a short arm, and a telomere are required; however this rearrangement was shown to occur in the dissociation that formed the two chromosomal races of the morabine grasshopper K. scurra.
Whole-Arm Interchanges
Whole-arm interchanges occur when chromosomal breaks and rejoinings near the centromeres of metacentric chromosomes result in the exchange of whole chromosome arms (Fig. 3) . It has been noted that such exchanges distinguish races and species more frequently than reciprocal translocations, perhaps because the former maintain a sequence of coadapted genes in the arms concerned.
Complex Rearrangement
Complex rearrangements such as insertions, involving three or more breaks, have been noted in insect chromosomes, particularly after damage induced by radiation. Such work, particularly by H. Muller in D. melanogaster, led to each arm of the chromosome being defined as oriented from the centromere to the telomere.
CLASS ARACHNIDA
Spiders
Y chromosomes are absent in spiders, so sex appears to be determined by genic balance of the X chromosomes and the autosomes, as in most insects. White (1973) illustrated 175 species with X1X20 males and X1X1X2X2 females with female haploid numbers of n = 8-24, plus an outlier with 48. There is also 26 species with X0 male, XX female sex-determination and femalen = 4, 11-14, plus 12 species with X1X2X30 male/X1X1X2X2X3X3 female with femalen = 14-24,both of which are regarded as derived from the more-numerous X1X20/X1X1X2X2 species. In male spiders multiple X chromosomes migrating to the same pole during anaphase I of meiosis are closely-associated, but they do not form chiasmata. In females with multiple X chromosomes each pair forms separate chiasmate bivalents in the few cases observed.
The chromosomes of spiders are monocentric and mostly all-acrocentric, with single chiasma near the centromere in the long arm in the autosomes of males; by contrast, the chiasmata in the few female spiders investigated appear to be distal. The slight correlation of increasing numbers of autosomes with increasing numbers of X chromosomes, and other more extreme changes in chromosomes number, is not regarded as due to polyploidy. Somewhat surprisingly, considering their peculiar sex chromosomes and mating habits, parthenogenesis (thelytoky) is unknown in spiders.
Scorpions
In scorpions the heterogametic sex is not known, so the determination of sex may not be chromosomal.
In the Family Buthidae, which was separated from the rest of the families of scorpions as early as the Silurian, the haploid numbers of chromosomes are very low to moderate: n = 3-12. In these scorpions, particularly genus Tityus, the bivalents in male meiosis appear to be holocentric and achiasmate, but this may be due to the unusual nature of meiosis in this genus. In one other buthid the chromosomes have been seen to be monocentric in mitosis, which is probably the rule. Also, in Tityus there is evidence of almost all forms of chromosomal rearrangement with some individuals showing odd diploid numbers indicating heterozygosity for rearrangements. One species, T. serrulatus, is a diploid thelytokous parthenogen.
The non-buthid families of scorpions show higher haploid numbers, n = 18-56, and the chromosomes in these families are clearly monocentric, with the centromeres usually sub-terminal.
Mites and Ticks
Mites and Ticks, subclass Acari, mostly have low chromosome numbers only a little above the minimum of n = 3. In 5 families there are diploid heterogametic males showing X0/XX sex-determination with the one genus showing XY and X1X2Y males regarded as derived from the X0 forms. Meiosis in some of the diploid males is achias-matic. In 5 other families of mites and ticks haplo-diploidy pertains. All of these mechanisms indicate a very low index of recombination in mites and ticks.
The question of centricity in mites and ticks is poorly resolved, with one species of water-mite, Eylais setosa, proven to have holo-centric chromosomes.
In the mites and ticks numerous thelytokous forms have been recorded; this contrasts with the low frequency of thelytoky in spiders and scorpions.
