1. Introduction
Since virtually all cells of an individual animal contain the same genetic information, the diversity in differential gene activity required for the development and formation of specialized cells must be ensured by mechanisms that do not involve changes in genomic sequence while efficiently activating or silencing certain genes. These “mitotically and/or meiotically heritable changes in gene function that cannot be explained by changes in DNA sequence” (Russo et al., 1996) are referred to as epigenetic, and include the modification of the genome by DNA methylation, the modification of histones, and the adoption of specific chromatin structures (Jaenisch and Bird, 2003; Reik etal., 2003). Although epigenetic modifications normally establish a stable cell identity, this cellular memory can be erased or altered. The development of clones from differentiated nuclei establishes that the ooplasm can create a substitute for the zygotic epigenotype from a somatic cell nucleus. Normally, the genome is “reset” during germline development, such that the gametic genomes are prepared to execute a developmental program once the fertilization and remodeling into a zygotic genome has occurred (Renard, 1998).
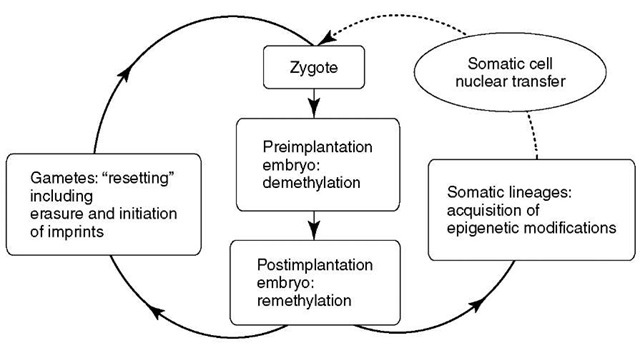
Germline reprogramming also involves the erasure and establishment of genomic imprints that regulate parent-of-origin-dependent gene expression (Mann, 2001). Apparently, exposure of a somatic nucleus to the ooplasm is sufficient to remodel its epigenetically distinct phenotype to one that is similar enough to that of the zygote, permitting development while bypassing reprogramming events that occur in the germline (Figure 1). Poor development, survival, frequent defects, and gene expression errors prevalent in mammalian clones (Ogura etal., 2002; Rideout etal., 2001; Wakayama and Yanagimachi, 1999b; Wells, 2003), and possibly the diminishing efficiency when cloning from clones for multiple generations (Wakayama et al., 2000), suggest that epigenetic reprogramming of somatic cell nuclei in clones is usually flawed or incomplete.
2. Genomic imprinting
The failure of clones, and the abnormalities observed in those that develop, are presumably due to incomplete reprogramming that affects gene expression. Clones exhibit gene expression abnormalities at several developmental stages (Daniels etal., 2000; Daniels etal., 2001; Humpherys etal., 2002; Inoue etal., 2002; Wrenzycki et al., 2001) including essential embryonic genes (Boiani et al., 2002; Bortvin et al., 2003). Genes subject to parental allele-specific imprinting have been considered key to the problems in clone development, as loss of the parental-specific imprint would require the germline passage to be reestablished (Jaenisch, 1997). Allelic expression of imprinted genes is regulated by parental-specific imprinting marks that are set in the germline, some of which involve differential methylation of regulatory regions. Dysregulation of imprinted genes has severe consequences on development, apparent in the early death of uniparental embryos (Barton etal., 1984; Mann and Lovell-Badge, 1984; McGrath and Solter, 1984; Surani et al., 1984), the abnormalities observed in those with uniparental duplications of chromosomal regions (Cattanach, 1986) and targeted disruption of imprinted gene expression (DeChiara etal., 1990; Eggenschwiler etal., 1997; Lau etal., 1994; Leighton et al., 1995). Abnormalities associated with disruption in the expression of imprinted genes regulating fetal growth, including insulin-like growth factor 2 (Igf2) and Igf2 receptor (Li et al., 1998; Reik and Maher, 1997), are similar to some of the most common phenotypes of clones: respiratory failure (Hill etal., 1999; Ogura etal., 2002; Wells, 2003), fetal overgrowth (Young etal., 1998), increased birth weight (Eggan etal., 2001), and placental hypertrophy (Hill etal., 2000; Humpherys etal., 2001; Ono etal., 2001; Tanaka etal., 2001; Wakayama and Yanagimachi, 1999a; Wakayama and Yanagimachi, 2001). Several imprinted genes are indeed expressed at abnormal levels in clones both at fetal or perinatal stages, but particularly so in the placentae (Humpherys et al., 2002; Inoue et al., 2002). The imprinted genes commonly implicated in the dysregulation of fetal growth, Igf2, Igf2r, and H19, are, however, expressed at normal levels and presumably not implicated (Humpherys et al., 2002; Inoue et al., 2002; Wells, 2003). Additionally, the abnormal expression levels observed in several imprinted genes analyzed were not associated with an imbalance in the allele specificity (Humpherys et al., 2002; Inoue et al., 2002). Therefore, the aberrant expression of imprinted genes seems to be stochastic with no apparent correlation to phenotypes, at least in those clones that develop to midgestation or later. As this proportion of clones represents a minority of those generated, it does not preclude that dysregulation of imprinted genes contributes to the death of the large high proportion of clones that occurs early in gestation.
Figure 1 Somatic cell nuclear transfer bypasses germline reprogramming
Analysis of clones at early developmental stages, such as the blastocyst, allows a more representative analysis of epigenetic and gene expression changes in the majority of clones, presumably indicative of reprogramming. In the mouse, most clones at the blastocyst stage retain or emulate the allele specificity of expression of imprinted genes with monoallelic expression patterns in both somatic cells and the early embryo, such as H19, Meg3, or Snrpn (Mann etal., 2003). This may reflect either continuation or a correct reestablishment of the allele-specific imprint. However, the differential methylation patterns of control regions involved in the regulation of allelic expression appear not to be established correctly for H19 and Snrpn. This may not influence preimplantation stage expression but could predict the loss of allele-specific expression in postimplanation development. For two autosomal imprinted genes that differ in expression between preimplantation stage and the soma, Ascl2 and Igfr2, being expressed biallelically in the early embryo but monoallelically in somatic cells, expression patterns are random with both mono- and biallelic expression patterns in clone mouse blastocysts. This suggests that reprogramming required for regulation of allele-specific expression often fails after somatic cell nuclear transfer. The proportion of clones at the blastocyst stage with correct reprogramming/expression of autosomal imprinted genes is very low (4%; Mann et al., 2003). Methylation and expression of imprinted genes are, however, altered in embryos due to culture in vitro and possibly have long-term effects on development (Doherty etal., 2000; Khosla etal., 2001; Young etal., 2001). Clones require considerable periods of in vitro culture, and are apparently not an exception to this phenomenon (Mann et al., 2003).
3. X inactivation
In contrast to autosomal imprinted genes, faithful recapitulation from mono- to bial-lelic activity has been observed in mouse clones with respect to X inactivation. The inactive X from a female somatic donor nucleus becomes activated in clones during preimplantation stages, followed by random X inactivation in the epiblast (Eggan et al., 2000). Since nonrandom inactivation is observed in the trophectoderm lineage of midgestation clones, with preferential inactivation of the previously inactive X chromosome of the somatic donor nucleus, it appears that an X chromosome silenced randomly in the epiblast carries an imprinting mark that is functionally equivalent to that of the paternal X chromosome of the zygotic genome. Placental tissue of perinatal bovine clones exhibited preferential inactivation of one X chromosome in viable clones, contrasting with biallelic activity in clones that died at birth, suggesting a similar recognition process in bovine clones but also a possible correlation of biased X inactivation in extraembryonic tissues with viability (Xue et al., 2002). However, as cloning of mammals from female versus male somatic cells is similar in efficiency, X inactivation is apparently not a limiting factor in clone development (Heyman et al., 2002; Kato et al., 2000).
4. Global methylation changes in clones
The developmental stages subsequent to the stage at which nuclear transfer is performed, normally include a sequence of major changes in the methylation status of the zygotic genome. In murine and bovine embryos, the paternal genome appears to be rapidly demethylated within hours of fertilization (“active” demethylation), while demethylation of the maternal genome occurs in a replication-dependent manner during cleavage stages (“passive” demethylation) (Dean et al., 2001; Mayer et al., 2000; Oswald et al., 2000; Rougier et al., 1998). Global de novo methylation of the hypomethylated embryonic genome occurs at late preimplantation stages and after implantation. The recapitulation of stage-specific methylation patterns in somatic cell clones of several species is inconsistent, which can be interpreted as errors in reprogramming. Analysis of global methylation of genomic repetitive elements in the early cleavage (Bourc’his et al., 2001), morula (Dean et al., 2001), and blastocyst (Kang et al., 2001) stage, bovine clones revealed abnormally high levels of methylation, similar to those of the somatic donor nuclei. While observations differ in respect to conservation (Dean et al., 2001) or absence (Bourc’his et al., 2001) of an initial wave of active demethylation, there is a consensus that passive demethy-lation during early cleavage stages is lacking (Kang etal., 2001). Faithful recapitulation of preimplantation-stage-specific methylation changes occurred on one single-copy sequence analyzed (Kang et al., 2003) but not on satellite sequences (Bourc’his et al., 2001; Kang et al., 2001; Kang et al., 2002). These contrasts in methylation for different sequences have been ascribed to the structural differences between permanent (satellite sequences) and facultative (unique sequences) hete-rochromatin (Kang et al., 2003). Interpretation of methylation status changes at the early developmental stages cannot predict developmental outcomes, however, the widespread dysregulation of global methylation, particularly in the trophectoderm of bovine clone blastocysts (Kang et al., 2002), is consistent with the overall and, in particular, the placental gene expression abnormalities in clones at later developmental stages. Some variation from the normal state of DNA methylation is compatible with postnatal development (Humpherys et al., 2001; Ohgane et al., 2001). However, major level differences in global methylation coincide with loss of viability, as evident from null mutants for DNA methyltransferase in the mouse (Jaenisch and Bird, 2003) and the observation of genome-wide hypomethylation in the majority of aborted bovine clone fetuses at late gestational stages (Cezar et al., 2003).
5. Conclusions
The abnormal or failing development of mammalian somatic cell clones presumably reflects inappropriate gene expression, precipitated by failure to put in place the necessary epigenetic information. It should also be considered that the cloning efficiency using donor nuclei from less-differentiated cells such as embryonic blas-tomeres and cells is low, and possibly limited by factors other than reprogramming of gene expression (Cheong et al., 1993; Eggan et al., 2001; Otaegui et al., 1994; Prather etal., 1987; Rideout etal., 2000; Tsunoda and Kato, 1997; Willadsen, 1986).
Reprogramming of the somatic cell nucleus is typically imperfect, evident at both the level of expression and in the epigenetic modifications of imprinted genes but is possibly due to the retention of characteristics of the somatic cell, at the exclusion of, or conflicting with normal developmental epigenetics. Additional evidence for the maintenance of the epigenome of the donor nucleus stems from studies indicating retention of somatic cell-like metabolism (Chung et al., 2002) and gene expression (Gao et al., 2003). As the abnormal phenotypes observed in clones coincide with abnormal gene expression but do not necessarily correlate with imprinted gene expression, reprogramming errors are apparently genome-wide (Humpherys et al., 2002). This is consistent with the finding that many genes are downregulated in clones. The threshold at which these differences prevent development are unknown, and gene expression and methylation variation in viable clones at term exemplifies that development is somewhat tolerant to variation in epigenetics. To assess the quality of reprogramming, it will be essential to define those epigenetic criteria that not only need to be reset after nuclear transfer but also correlate with viability.