1. Introduction
Gametogenesis and early embryogenesis in mammals are under the control of genetic and epigenetic mechanisms. A remarkable aspect of epigenetics is the reprogramming of allele-specific gene expression by DNA methylation and histone modifications (acetylation, phosphorylation, methylation, and ubiquitylation). A disruption of these two biochemical events leads to abnormal developmental processes, including Prader-Willi (PWS) and Angelman (AS) syndromes, and the Beckwith-Wiedemann syndrome (see Article 29, Imprinting in Prader-Willi and Angelman syndromes, Volume 1 and Article 30, Beckwith-Wiedemann syndrome, Volume 1) (see Nicholls and Knepper, 2001 for a comprehensive review).
Two clinically related issues are relevant to the epigenetic reprogramming and genomic imprinting in germ cells and preimplantation embryos. First, advances in assisted reproductive technology as an approach to treating infertility have attracted attention to the potential risk of birth defects when major epigenetic events can be disrupted when round spermatids and preimplantation embryo are developed or maintained in culture, respectively (Lucifero etal., 2002; Gosden etal., 2003). Second, prospects of epigenetic therapy based on inhibitors of enzymes controlling epigenetic modifications, in particular, DNA methyltransferases and histone deacetylases, have opened the possibility that genes that have undergone abnormal epigenetic silencing may be reactivated (Egger et al., 2004). This brief review summarizes current knowledge on the developmental occurrence of genomic imprinting during gametogenesis and in the preimplantation embryo. Knowledge of these highly timed events can contribute to implementing safe and efficient assisted reproductive technologies.
2. Components of the epigenetic reprogramming machinery
DNA methylation and histone modifications are epigenetic heritable changes functioning as efficient modulators of transcription. ATP-dependent chromatin modifications contribute to DNA methylation and histone modification events (see Reik etal., 2001; Li, 2002 for comprehensive reviews). DNA methylation occurs at CpG dinucleotides and is catalyzed by DNA methyltransferase 1 (Dnmt1), a maintenance enzyme operating after DNA replication, and Dnmt3a and Dnmt3b, both required for de novo DNA methylation patterns during development. Both Dnmt1 and Dnmt3a interact with histone deacetylases (HDACs) to repress transcription. CpG-binding proteins, with a methyl-CpG-binding domain (MBD), recruit different chromatin-remodeling proteins and transcription regulatory complexes to DNA-methylated regions in the genome. Histone modifications occur at the lysine, arginine, and serine residues located at the amino-terminal tail of histones. Histone methyltransferases include H3-K4 methyltransferase (methylation of lysine 4 of Histone 3; associated with active gene expression), H3-K9 (methylation of lysine 9 of Histone 3; associated with transcriptional silencing), and five H3-K9 methyltransferases (Suv39 h1 and Suv39 h2, G9a, ESET/SetDB1 and Eu-MHTase1). Several HDACs have been identified, including transcription coactivators with intrinsic histone acetyltransferase activity. ATP-dependent chromatin-remodeling protein complexes (SWI/SNF/Brm, ISWI, and Mi-2/NuRD) use ATP hydrolysis to make nucleosomal DNA and the histone core accessible to DNA methylation and histone modifications.
3. Epigenetic reprogramming in germ cells
The most differentially methylated regions in imprinted genes of primordial germ cells (PGC) located in the genital ridge become demethylated or “erased” by embryonic day 13 to 14 in both females and males. Prior to this (embryonic days 11.5 or 12.5), PGC are highly methylated and H19 (a paternally imprinted gene) and Igf2r (insulin-like growth factor 2 receptor gene, a regulator of fetal growth and embryonic development) display normal imprinting patterns. Both genes are more methylated in cells with an XY chromosome complement than those with an XX chromosome complement (Durcova-Hills et al., 2004). Following demethylation, male PGC in the testicular cords enter mitotic arrest and primary oocytes, surrounded by follicular cells in the fetal ovary, become arrested at the end of meiotic prophase. During spermatogenesis, epigenetic changes of the spermatocyte lineage and the derived postmeiotic haploid spermatids have enabled the use of nuclei of in vitro developed spermatids from spermatocytes precursors to generate normal offspring when injected into mature oocytes (Marh et al., 2003).
Genomic imprinting of the sperm and egg genomes is regulated by differential methylation, an activity dependent on DNA methyltransferases. Recent studies of the Dnmt3-Like (Dnmt3L) gene have shown that the encoded protein shares homology with Dnmt3a and Dnmt3b in the PHD-zinc-finger domain, but lacks both the highly conserved methyltransferase motifs and enzymatic activity. Dnmt3L-deficient females generate mature and functional oocytes, but derived embryos have neural tube and placental abnormalities and are nonviable by mid-gestation. Analysis of DNA methylation patterns of Dnmt3L-deficient oocytes shows that genes on different chromosomes (Igf2r, Mest (mesoderm-specific transcript) and Peg3 (paternally expressed gene 3) and several genes in the Snrpn (a maternally imprinted gene) locus) fail to display epigenetic maternalization and the monoallelic expression of all maternally imprinted genes is thought to be lost in the offspring.
The Dnmt3L protein interacts with the Dnmt3 family of DNA methyltransferases and might cooperate with Dnmt3a or Dnmt3b to regulate gamete-specific methylation of imprinted genes in oocytes. In fact, Dnmt3a/Dnmt3b-deficient oocytes also fail to establish epigenetic maternalization. Dnmtl -deficient oocytes can establish methylation imprints but cannot maintain imprinting in preimplantation embryos. Therefore, it appears that Dnmtl is essentially a housekeeping methyltransferase required for maintaining tissue-specific methylation patterns. High levels of the ovarian Dnmtlo accumulate in the oocyte nuclei during the follicular growth phase, when genomic imprinting has been established. Like Dnmtl, Dnmtlo is a housekeeping DNA methyltransferase.
In the male, Dnmt3L gene deficiency results in spermatogenesis arrest and sterility due to spermatogonia entering meiosis and being killed by an asynapsis checkpoint just prior to pachytene (Bourc’his and Bestor, 2004). In the female, in contrast, mature and functional oocytes are produced in the Dnmt3L deficient mutant. Dnmt3a knockout male mice display testes with abnormal meiotic prophase spermatocytes and few mature sperm. In addition to DNA methylation, histone modifications are critical for spermatogenesis. Histone methyltransferase Suv39h1 and Suv39h2 -deficient mice are sterile because of meiotic arrest at the pachytene stage of meiotic prophase. These examples emphasize the impact of epigenetic modifications in male and female fertility.
DNA methylation and histone H3-K9 methylation during spermatogenesis correlate with histone deacetylation. Somatic histones are hyperacetylated in spermato-gonia and in pre-leptotene spermatocytes but acetylated histones are not detected from leptotene on and in round spermatids. It appears that DNA methylation and histone modifications play a role in modulating meiotic chromosome architecture and in ribosomal and nonribosomal transcription activity. In summary, the timing and extent of remethylation following methylation erasure in PGC is slightly different during oogenesis and spermatogenesis (Figure 1). In the male, remethylation begins in prospermatogonia (gonocytes) by embryonic day 15 to 16 and continues throughout spermatogenesis. Therefore, remethylation takes place before mitotic amplification of the spermatogonial stem cell lineage at the time of puberty. Two representative genes, H19 and Mest, display developmental epigenetic paternalization: both genes are unmethylated in fetal prospermatogonia, Mest remains unmethylated throughout spermatogenesis, and H19 methylation first appears in spermatogonia and is maintained throughout spermatogenesis (Kerjean et al., 2000). In the female, remethylation is observed during the growth of oocytes, a prolonged developmental process enabling sequence methylation at different time points. Although germ cell-specific DNA methyltransferases have not been identified, Dnmt3a and Dnmt3b are good candidates (Kaneda et al., 2004).
4. Epigenetic reprogramming in preimplantation embryos
After fertilization, the chromatin of the paternal genome undergoes changes consisting in the replacement of protamines by acetylated histones and, from some evidence, DNA demethylation by an active mechanism that is completed before DNA replication. Some sequences in the paternal chromosomes are protected from demethylation, in particular, the imprinted genes H19 and RasGrfl. The maternal genome is demethylated by a passive mechanism dependent on DNA replication. Dnmt1 is not present in the nucleus and, therefore, passive demethylation occurs. In the eight-cell embryo (mouse), Dnmt1 reappears in the nucleus. At the time of implantation, both the paternal and maternal genomes are remethylated with the participation of Dnmt3a and Dnmt3. Variations in the methylation of imprinted genes in embryonic and extraembryonic cell lineages are characteristic. The postzy-gotic demethylation and remethylation sequence (mimicking to some extent the reprogramming saga of the germ cell lineage) presumably removes epigenetic modifications acquired during gametogenesis. An important issue is the consequence of using somatic nuclei from adult and embryonic donors during animal cloning. Somatic donor nuclei contain a highly methylated genome, a departure of the precise postzygotic demethylation-remethylation timely sequence. Although somatic nuclei are reprogrammed in clones, the timing and efficiency of demethylation-remethylation of genes critical for cellular differentiation may differ, thus leading to developmental abnormalities and lethality.
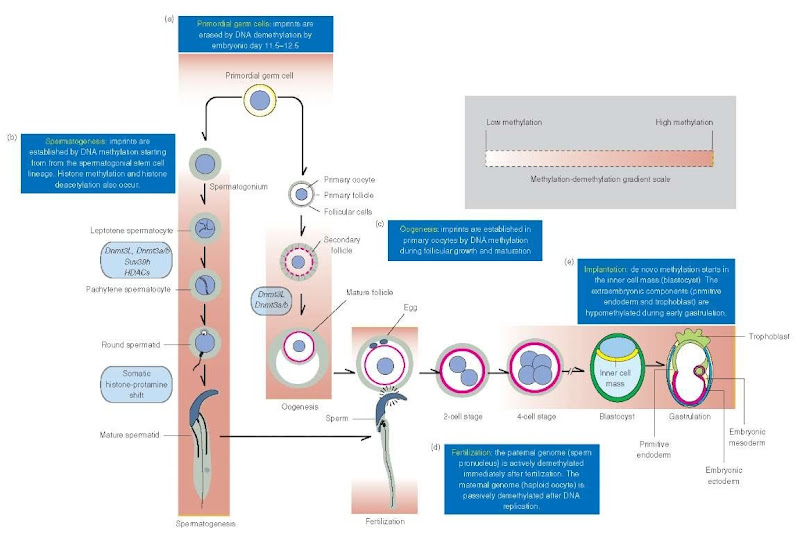
Figure 1 Epigenetic reprogramming during gametogenesis and early embryo implantation (mouse), (a) Demethylation at imprinted loci erase parental imprinting marks in primordial germ cells by embryonic day 11.5-12.5. (b) During spermatogenesis, methyltransferases Dnmt3a and Dnmt3b, in association with Dnmt3L, start to reestablish paternal methylation from spermatogonia on. In addition, histone hypoacetylation-deacetylation – controlled by histone deacetylases (HDACs) – and histone methyltransferases. including Suv39h, regulate chromatin organization and transcription activities. During spermiogenesis, somatic histones are gradually replaced by transient basic proteins and finally by protamines. Consequently, the nucleosomal beaded chromatin pattern is replaced by smooth chromatin fibers and the genome becomes transcriptionally silent, (c) During oogenesis, maternal-specific genomic imprints are reestablished in the DNA of the oocyte starting during follicular growth and continuing throughout follicle maturation by the de novo activity of methyltransferases Dnmt3a, Dnmt3b, and Dnmt3L. (d) Immediately after fertilization, the paternal genome (sperm pronucleus) in the zygote is demethylated by an active mechanism. Demethylation of the maternal genome (egg pronucleus) takes place by way of a passive mechanism after DNA replication has occurred. Chromatin decondenses and transcription activities of the zygote, essential for early development, take place. Most of the methylation marks inherited from the male and female gametes are erased by the blastocyst embryonic stage (embryonic day 3.5). (e) During early implantation, the embryonic DNA methylation patterns are established in a lineage-specific manner by de novo methylation starting in the inner cell mass of the blastocyst. DNA methylation levels increase in the primitive ectoderm. The DNA of extraembryonic cells (primitive endoderm and trophoblast) remains hypomethylated. Specific parental imprinted genes protected from demethylation are not indicated for clarity.