Phosphorylation of Nav1.5
Phosphorylation is a well-known regulatory mechanism of ion channels, often resulting in altered biophysical properties. Protein kinase C (PKC) activation provokes a drastic reduction in Nav1.5 current amplitude as well as a negative shift in steady-state inactivation (Qu et al., 1994). This effect is believed to be primarily mediated through the phosphorylation of serine residue 1503 (Murray et al., 1997; Qu et al., 1996). The function of glycerol 3-phosphate dehydrogenase 1-like (GPD1L) has recently been linked to the PKC phosphorylation of Nav1.5 (Valdivia et al., 2009). GPD1L catalyses the conversion of glycerol-3-phosphate to dihydroxyacetone phosphate. Glycerol-3-phosphate stimulates -through several intermediate proteins – PKC and thereby feeds the PKC-mediated phosphorylation of Nav1.5. Mutations in GPD1L have been associated to Brugada (London et al., 2007; Weiss et al., 2002) and sudden infant death syndromes (Van Norstrand et al., 2007), and Valdivia and colleagues have shown this to be related to the decreased activity of GPD1L, inducing higher PKC activity and a reduced sodium current (Valdivia et al., 2009). The tyrosine phosphorylation of Nav1.5 has also been found to promote changes in the channel kinetics. The cardiac-expressed protein kinase Fyn induces a depolarising shift in steady-state inactivation (Ahern et al., 2005). By mutating tyrosine residue 1495, located in the linker between domains 3 and 4 and in close proximity to residues involved in inactivation (Patton et al., 1992), the authors found the effect of Fyn to be abolished. In contrast, the expression of the protein tyrosine phosphatase PTPH1 – which is also expressed in the heart – induced a hyperpolarisation shift in steady-state inactivation (Jespersen et al., 2006). PTPH1 interacts with the 14-3-3p regulatory protein suggest that 14-3-3 p functions as a regulator or adapter protein of the phosphatase (Zhang et al., 1997). Another member of the 14-3-3 family, namely 14-3-3r|, has been found to interact with the Nav1.5 cytoplasmic I inter-domain, modifying the biophysical properties of the channel (Allouis et al., 2006). Whether or not this interaction modulates the level of Nav1.5 phosphorylation is unknown.
Plasma membrane stability of Nav1.5
Nav1.5 holds a C-terminal PDZ domain-binding motif. This domain binds syntrophin, which again interacts with dystrophin (Gavillet et al., 2006). The most prominent role of dystrophin is to provide a structural link between the cytoskeleton and the extracellular matrix in order to maintain muscle integrity. However, experiments performed by Abriel and co-workers on dystrophin-deficient mdx mice indicated the cardiac sodium channel to be regulated through a syntrophin/dystrophin complex (Gavillet et al., 2006). A significant reduction in Nav1.5 protein and current levels – together with ECG alterations – was found when the hearts from these mdx mice were analysed. The functional importance of this interaction has been confirmed in humans, where mutations in a1-syntrophin have been associated with long QT syndrome and sudden infant death syndrome (Cheng et al., 2009; Ueda et al, 2008; Wu et al, 2008).
For an increasing number of ion channels, Nedd4/Nedd4-like ubiquitin-protein ligase mediated internalisation has been found to be important (review by Abriel & Staub, 2005). This class of protein ligases – counting 9 members – interacts with membrane proteins holding a PY-motif (Staub et al., 1996). Ubiquitin is a 76 amino acid protein which can be covalently linked to lysine residues on target proteins, marking them for internalisation, followed by either degradation or intracellular storage (Hershko & Ciechanover, 1998; Hicke, 1999). Nav1.5 is regulated by Nedd4/Nedd4-like mediated ubiquitylation (Rougier et al., 2005; van Bemmelen et al., 2004). In vitro electrophysiological experiments revealed that a down-regulation in current density – without altering the biophysical properties – to be was induced by Nedd4-2 through a PY-motif located in the C-terminal tail of Nav1.5. Nedd4-2 induces an increase in the ubiquitylation of Nav1.5, which leads to a drastic redistribution, where Nav1.5 proteins are almost absent from the surface membrane but are instead found in intracellular compartments.
Other sodium channels in the heart
Although Nav1.5 is the most important sodium channel in the heart, other voltage-gated sodium channels may also play a role in generating the cardiac INa. Neuronal sodium channels do, in contrast to Nav1.5, have a very high sensitivity to tetrodotoxin. This has been used to investigate the potential function of neuronal voltage-gated sodium channels in the heart. Although present at a relatively low mRNA level (Gaborit et al. , 2007) neuronal sodium channels have been suggested to play a role in electrical-chemical coupling, as low tetrodotoxin concentrations lead to a reduction in sercoplasmic reticulum calcium release (Torres et al., 2010) and thereby reduce left ventricular functioning (Maier et al., 2002). Brette & Orchad found that TTX-sensitive INa makes up approximately 15% of the total INa in isolated rat ventricular cells, which decreased the rate of the depolarisation of the action potential by 10% (Brette & Orchard, 2006). Further, the sodium current in Purkinje fibres has been shown to be sensitive to low concentrations of tetrodotoxin (Carmeliet, 1987), indicating that the neuronal sodium channels participate in the propagation of the cardiac action potential.
Recently, genome-wide association studies have revealed that SCN10A – encoding the Nav1.8 sodium channel – seems to participate in determining the conduction velocity in both atria (PR interval) and the ventricles (QRS duration) (Chambers et al., 2010; Holm et al., 2010; Pfeufer et al., 2010). Nav1.8 has a low sensitivity to tetrodotoxin, as with Nav1.5, and it can therefore be speculated that this channel has been overlooked up until now.
L-type calcium channels
The fast depolarisation (phase 0) driven by the influx of sodium through the voltage-gated sodium channels triggers the activation of voltage-gated calcium channels. Both voltage-gated T-type and L-type calcium channels have been reported to be expressed in the heart. The T-type channels are low voltage-activated transient Ca2+ channels which are functionally expressed during development, while they are drastically down-regulated in adult myocytes (Ono & Iijima, 2010). However, these T-type calcium channels may still play a role in impulse generation in the sinoatrial node (Hagiwara et al., 1988). The long lasting, high voltage-activated L-type Ca2+ channels are both abundant and ubiquitously expressed in the heart (Bodi et al., 2005). These voltage-dependent calcium channels (VDCC) bind dihydropyridine and have, therefore, also been named dihydropyridine receptors (Taira et al., 1987; Tanabe et al., 1987). The L-type Ca2+ channels are the primary source of extracellular calcium influx. The opening of L-type Ca2+ channels is delayed when compared with Na+ channels and in contrast to the voltage-gated sodium channels, the L-type Ca2+ channels inactivate slowly (<100 ms) in a voltage- and calcium-dependent manner (Bean, 1985). This slowly inactivated calcium current is – together with the fine-tuned regulation of sodium and potassium conductance – the basis for the action potential plateau observed in ventricular myocytes (phase 2). The ryanodine receptor calcium channels (RYR2) – which are located in the sarcoplasmic reticulum in close proximity to the L-type Ca2+ channels – is activated by the calcium influx (Bers, 2004). This RYR2-mediated sarcoplasmic calcium release is the major contributor in the activation of the contractile machinery (Bers, 2002). The cardiac L-type calcium channel consists of a pore-forming a-subunit, the Cav1.2 protein, which is encoded by Cacna1c. Cav1.2 has a similar topology to Nav1.5 (Fig. 3). A functional cardiac channel complex is composed of four polyproteins which, apart from Cav1.2, form the p and a2/ 8 auxiliary subunits (Bodi et al., 2005). The a2 and 8 subunits are encoded by the same gene and are separated by proteolytic cleavage (De Jongh et al., 1990). Several different isoforms of this protein are known. The a2/8 subunits are linked together by a disulphide bridge and are closely associated with the Cav1.2 a-subunit by surface interaction. The a2 subunit is entirely extracellular, and the 8 subunit has a single transmembrane region with a very short intracellular part. The a2/8 subunits have been suggested to increase the membrane density of the channel complex, and mice lacking this gene have a tendency to have bradycardia (Ivanov et al., 2004). All four calcium channel p-subunits (CACNB1-4) are known to modify the currents; however, it has been suggested that p2 is the primary subunit in the heart (Colecraft et al., 2002). The p-subunits play a prominent role in the trafficking of the channel complexes to the cell surface membrane (Bichet et al., 2000; Chen et al., 2004; Van et al., 2004). Furthermore, the absence of p-subunits renders the channel insensitive to p-adrenergic stimulation (Mikala et al., 1998). One of the important regulatory mechanisms of L-type calcium channels is cAMP-dependent phosphorylation, which increases the amplitude of the calcium current (McDonald et al., 1994). An increase in cAMP is induced by the p-adrenergic control of cardiac functions. p-adrenergic stimulation thereby leads to an increased calcium influx through the L-type channels, which facilitates an increased calcium release from the ryanodine receptors. Other important regulators of L-type calcium channels are calmodulin-dependent protein kinase II (CaMKII) (Maier & Bers, 2007) and calcium-induced inactivation through binding to calmodulin (Bodi et al., 2005).
Potassium channels
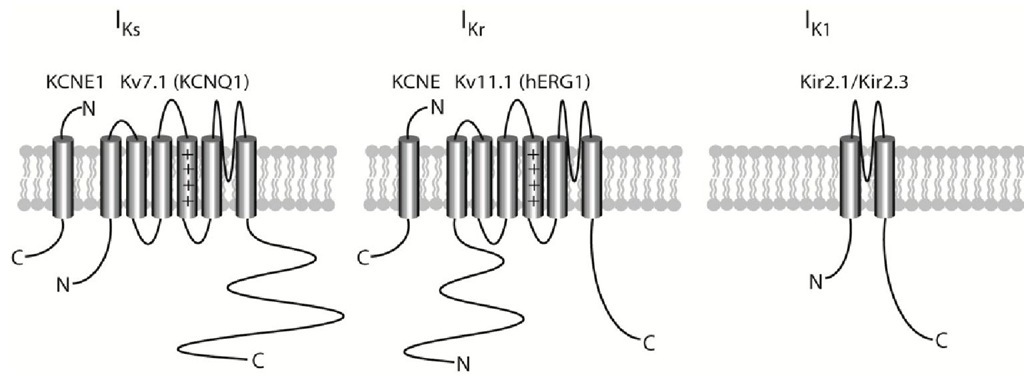
In the heart, potassium conductance is conducted through a number of different potassium channels. All of the potassium channels described below consist of six transmembrane domains – except for Kir2.x which has two – and assembles into tetrameric complexes, which can either be homo- or heteromeric (Nerbonne & Kass, 2005) (Fig. 4). In the early phase of the action potential, the transient outward potassium current (ITo) is important in the atria and in subepicardial ventricular myocytes. The ultra-rapid potassium current (IKur) – which is also a fast activating current present early on in the action potential – is predominantly expressed in the atria. The rapid and slow delayed rectifier potassium currents, IKr and IKs, respectively, are, together with the inward rectifier current IK1, the primary currents responsible for repolarising the myocyte membranes in the final part of the action potential and thereby terminating it (phase 3). All three of these currents are important in both atria and the ventricles.
Fig. 4. Topology of the major repolarising potassium channels and their P-subunits. 4.1 The transient outward (Kv4.x/Ito) potassium channels
The transient outward current ITo is composed of two different components, namely a calcium-dependent chloride current and a calcium-independent potassium current. While the molecular components underlying the chloride current are unknown, several recently published reports have revealed a detailed picture of the proteins involved in forming the potassium transient outward current (reviewed by Patel & Campbell, 2005). This current can be divided into a rapidly activating and inactivating current – named ITo,f – and a current with slow recovery kinetics – named ITo,s. ITo,s, conducted through Kv1.4 channels – which are regulated by KvP cytosolic proteins (Morales et al., 1995). Kv1.4 channels are expressed throughout the ventricular wall as well as in the atria, where it is suggested that they participate to a minor extent with ITo (Calloe et al., 2010; Calloe et al., 2011). The ITo,f channels activate rapidly (in the order of milliseconds) in a voltage-dependent manner and are inactivated through a somewhat slower process (in the order of tens of milliseconds). The pore-forming subunit in ITo,f which is predominantly present in larger mammals is Kv4.3, which when co-expressed with the Kv channel interacting protein 2 (KChiP2) recapitulates most of the features of the native current (An et al., 2000; Deschenes et al., 2002). Kv4.3 is homogenously expressed in the ventricle. The KChiP2 auxiliary protein potentiates the current conducted through the Kv4.3 channels by promoting cell surface expression. In fact, in human and canine ventricles, a transmural expression gradient of KChiP2 has been found to correlate with a much higher ITo,f in the subepicardial layer than in the subendocardial layer (Calloe et al., 2010; Deschenes et al., 2002; Gaborit et al., 2007; Soltysinska et al., 2009; Zicha et al., 2004). This large expression of ITo,f is responsible for the characteristic notch (phase 1 repolarisation) observed in subepicardial cardiomyocytes (Calloe et al., 2009; Di Diego et al., 2002). ITo,f is also prominently expressed in the atria, where it likewise participates in early repolarisation (Calloe et al., 2010; Calloe et al., 2011; Gaborit et al., 2007). While KChIP2 has the most prominent effect on Kv4.3 channels, with altered current levels as well as inactivation and recovery parameters (Patel & Campbell, 2005), other auxiliary subunits have also been shown to be important for ITo,f. Dipeptidyl aminopeptidase-related proteins (DPPs) affect the biophysical properties of the Kv4.3 channels in a manner very similar to KCHiP2 proteins, with the important difference that they also accelerate activation, thereby providing current properties resembling native ITo,f (Cotella et al., 2010; Nadal et al., 2003; Radicke et al., 2005). Kvp cytosolic proteins, which increase the expression of Kv4.3, have been suggested to regulate this transient outward potassium current (Yang et al., 2001). KCNE p-subunits, of which 5 different subtypes exit, have also been suggested to interact with Kv4.3/KCHiP2 channels as they modify the channel kinetics in in vitro studies (Radicke et al., 2006; Radicke et al., 2008). Recently, mutations in KCNE3 and KCNE5 have been linked to Brugada syndrome, which is a syndrome associated with an increased risk of ventricular fibrillation (Brugada & Brugada, 1992; Delpon et al., 2008; Ohno et al., 2011). Both KCNE3 and KCNE5 decrease the ITo current level when co-expressed, and as the mutations found in Brugada Syndrome patients provide an increase in current level compared to controls, it is suggested that this inhibitory effect of ITo is important in maintaining the current balance between the sodium and potassium currents in the early part of the ventricular action potential.
The ultra-rapid (Kv1.5/IKur) potassium channels
The ultra rapid potassium current IKur is well-expressed in the atria, where it contributes to repolarisation (Amos et al., 1996). This current activates early during an action potential and inactivates slowly. Hence, IKur is an important repolarising current throughout most of the atrial action potential. The molecular constituent of IKur is the Kv1.5 potassium channel (Wang et al., 1993). Although, IKur has predominantly been reported in atria, this current has also been suggested to play a role in canine and human ventricles (Calloe et al., 2010; Nielsen et al., 2007; Sridhar et al., 2007).
The fast delayed rectifier (hERG1/IKr) potassium channels
The rapid delayed rectifier current IKr is present in nodal tissue, atria, purkinje fibres and ventricles. The molecular correlate of IKr is the ether-a-go-go-related gene 1 product ERG1, also termed Kv11.1 (Sanguinetti et al., 1995; Trudeau et al., 1995). It is the unique biophysical features – with fast inactivation followed by slow deactivation – of the ERG1 potassium channel which makes it pivotal in cardiac repolarisation (Grunnet, 2010; Spector et al., 1996). Upon depolarisation, the ERG1 channels open but inactivate very quickly and at the same time display marked inward rectification (Grunnet et al., 2008b). This means that the ERG1 channel complexes conduct a minor potassium current during the initial depolarisation and the plateau phase of the cardiac action potential. However, when the membrane potential moves slightly towards the repolarisation potential – partly due to L-type calcium channel inactivation and partly due to IKs activation – then ERG1 channels are released from inactivation. As ERG1 channels only slowly progress into a closed state (deactivation) – and, therefore, are kept in an open state (Piper et al., 2005) – a relatively large potassium current is conducted and the membrane potential is accelerated towards the resting membrane potential. The inactivation of ERG1 channels is called C-type inactivation, which involves a change at the extracellular mouth of the pore modulated by the extracellular potassium concentration (Baukrowitz & Yellen, 1995). A low concentration of potassium will lead to a pore collapse. Hence, the external potassium concentration is an important regulator of potassium conductance, where low concentrations will reduce activity and high concentrations will increase activity. Loss-of-function mutations in hERG1 are associated with long QT syndrome type 2 (Sanguinetti et al., 1996a), while gain-of-function mutations have been found in short QT syndrome type 1 (Brugada et al., 2004; Cordeiro et al., 2005; Grunnet et al., 2008a).
Two splice variants of ERG1 have been reported. The originally identified ERG1 protein is termed ERG1a while an alternatively spliced variant, termed ERG1b, has a much shorter intracellular N-terminal with a unique 36 residue sequence (Lees-Miller et al., 1997; London et al, 1997). ERG1b displays different deactivation kinetics to ERG1a (Lees-Miller et al., 1997; London et al., 1997). The co-expression of mRNA levels corresponding to the levels found in the human ventricles of the two variants alter several of the kinetic parameters (Larsen et al., 2008), and this may explain a reported dispersion of IKr deactivation kinetics observed between myocytes isolated from the subepicardium and the mid-myocardium (Szabo et al., 2005). The membrane-spanning KCNE2 p-subunits have been found to modify the kinetics of the hERG1 channel (Abbott et al., 1999; McDonald et al., 1997). KCNE2/hERG1 expression in heterologous expression systems has been found to provide currents partly resembling native IKr, and as KCNE2 mutations found in long QT syndrome patients alter the channel properties it has been suggested that KCNE2 interacts with ERG1 in the heart (Abbott et al., 1999). However, another report has not found KCNE2 to act as an essential constituent of the ERG1 channel complex carrying native IKr (Weerapura et al., 2002).
The slow delayed rectifier (Kv7.1/IKs) potassium channels
The KCNQ1 gene, encoding Kv7.1 proteins, was cloned by Wang and co-workers using linkage analyses on genomic material from Long QT syndrome patients (Wang et al., 1996), and was, therefore, originally named KvLQT1. The voltage-gated Kv7.1 channel is progressively opened by increasing membrane depolarisations. The channel gives rise to slowly activating and deactivating potassium currents. Upon longer depolarising steps, a fraction of the KCNQ1 channels inactivate (Pusch, 1998). KCNQ1 potassium channels are expressed in several tissues throughout the body and regulate key physiological functions. The two most important roles of KCNQ1 channels are: i) the repolarisation of the cardiac tissue following an action potential, and ii) water and salt transport across epithelial tissues (reviewed by Jespersen et al., 2005).
The five relatively small one-transmembrane spanning KCNE proteins – KCNE1-5 – have been found to be highly promiscuous with respect to modulating the biophysical properties of Kv potassium channels as well as HCN pacemaker channels (McCrossan & Abbott, 2004). All five members of the KCNE family modify the properties of Kv7.1 channels (Jespersen et al., 2005). The co-expression of Kv7.1 with KCNE1 – formerly known as minK – recapitulates native IKs (Barhanin et al., 1996; Sanguinetti et al., 1996b), which not only plays a pivotal role in repolarising the myocardium but which is also important in transporting potassium across the strial marginal cells in the inner ear (Sunose et al., 1997). The co-assembly of Kv7.1 and KCNE1 results in an increase in single channel conductance, a positive shift in the voltage activation threshold, the slowing of activation and deactivation, and an almost complete absence of inactivation (Splawski et al., 1997). In long QT syndromes 1 and 5, which are caused by mutations in Kv7.1 and KCNE1, a reduced IKs current is observed (Wang et al, 1996; Wang et al, 1999).
IKs is the only potassium current which is upregulated with increased beating frequency. The upregulation of IKs is orchestrated by sympathetic mediated p-adrenergic receptor activation. The p-adrenergic receptor activation results in an increased level of cAMP and PKA stimulation, which interacts with the IKs channel complex through an A-kinase anchoring protein (AKAP) called ‘yotiao’ (Marx et al., 2002; Potet et al., 2001). PKA and protein phosphatase 1 interact with the C-terminal tail of KCNQ1 through yotiao, which leads to a phosphorylation of serine 27 in the N-terminus. cAMP-induced regulation of Kv7.1 is dependent on KCNE1 and Long QT mutations in both KCNQ1 and KCNE1 have been shown to disrupt this regulation (Kurokawa et al., 2004; Marx et al., 2002). The P-adrenergic activation increases the activation and slows the deactivation kinetics of IKs, and these features – together with the increased beating frequencies – have been suggested to underlie the profoundly augmented cardiac IKs current (Marx et al., 2002; Terrenoire et al., 2005). IKs is therefore essential for action potential shortening at increased beating frequencies. The importance of P-adrenergic stimulation is underlined by the fact that in humans IKs is almost absent without sympathetic stimulation (Jost et al. , 2005). KCNE2-5 P-subunits also interact with Kv7.1 channels, modifying the biophysical parameters (Angelo et al., 2002; Bendahhou et al., 2005; Grunnet et al., 2002; Jespersen et al., 2004; Mazhari et al., 2002; Tinel et al., 2000). Although KCNE2 is primarily believed to be of importance in the stomach, it has also been suggested as modifying IKs properties in the heart (Jiang et al., 2009; Wu et al., 2006). A polymorphism in KCNE4 has been associated with atrial fibrillation through a proposed gain-of-function mechanism (Ma et al., 2007), but solid evidence is still missing concerning a potential physiological function of the Kv7.1/KCNE4 interaction in the heart. KCNE5 expression drastically reduces the IKs current amplitude (Angelo et al., 2002). A KCNE5 mutation found in a patient with atrial fibrillation has been shown to increase IKs and it has therefore been suggested that KCNE5 P-subunits regulate the current conducted through Kv7.1/KCNE1 channels (Ravn et al., 2005; Ravn et al, 2008).
Under pathophysiological conditions, such as during ischemia, cell volume and pH may undergo considerable alterations. KCNQ1 channels have been found to be activated by a drastic increase in extracellular hyperosmolarity in cardiomyocytes (Sasaki et al., 1994; Vandenberg et al., 1996). In heterologous expression systems, it has been shown that hyperosmolar-induced swelling increases the Kv7.1 current while hyperosmolar shrinkage decreases the current (Grunnet et al., 2003). The ability of Kv7.1 to sense volume changes depends on an intact cytoskeleton which interacts with the N-terminal part of Kv7.1. As with volume changes, internal and external acidification also modifies the Kv7.1 current density. Homomeric KCNQ1 channels are inhibited by both intracellular and extracellular acidic pH (Freeman et al, 2000; Peretz et al, 2002; Unsold et al, 2000). KCNE P-subunits enforce differential effects on the Kv7.1 channel complex following acidification. While KCNE3 renders Kv7.1 insensitive to external acidification, KCNE2 induces an increase in the current level following such acidification, which seems to be determined by the extracellular and transmembrane domains of KCNE2 (Heitzmann et al., 2007). The pH-dependent regulation induced by KCNE1 has been disputed, as both a small decrease (Peretz et al., 2002) and an increase (Heitzmann et al., 2007) in current amplitude has been found; however, both external and internal acidification seem to modify the Kv7.1/KCNE1 current kinetics by changing the slow activation kinetics to an instantaneous onset (Heitzmann et al., 2007; Unsold et al, 2000).
The inward rectifier (Kir2.X/IKi) potassium channels
The resting membrane potential of cardiomyocytes - being between -80 and -90 mV – is close to the equilibrium potential of potassium, partly due to relatively large resting K+ conductance through inward rectifier potassium channels (IKir) (phase 4) (Dhamoon & Jalife, 2005). IKir channels are composed of four pore-forming subunits, being either homomeric or heteromeric and characterised by a preferentially conducting current at potentials below -50 mV (Lu, 2004). IKir is not, in contrast to the above described currents, voltage gated. The inward rectification profile, where much less current is passing when the membrane is depolarised than when it is repolarised, is not an inherent property of the channel protein itself, but reflects strong voltage dependence of channel block by intracellular cations, such as Mg2+ and polyamines (Ficker et al., 1994; Lopatin et al., 1994; Matsuda et al., 1987; Vandenberg, 1987). The primary inward rectifying current responsible for terminating the action potential – as well as for setting the resting membrane potential – is IK1, constituted by Kir2.1 and, to a lesser extent, the Kir2.2 and Kir2.3 proteins (Preisig-Muller et al., 2002; Zaritsky et al., 2001). Regional differences in the expression of IK1 have been described (Dhamoon et al., 2004; Samie et al., 2001) (Samie et al., 2001; Dhamoon et al., 2004) and the modulation of this current affects cardiac excitability and arrhythmogenesis (Nakamura et al, 1998; Plaster et al, 2001; Poelzing & Veeraraghavan, 2007; Warren et al, 2003). IK1 channels, such as ERG1 (IKr) channels, are regulated by extracellular potassium (Dhamoon et al., 2004; Hume & Uehara, 1985; Knot et al., 1996). Increased extracellular potassium augments potassium conductance – even though the potassium driving force is decreased – while a decreased concentration reduces the current. This biophysical property of IK1 and IKr channels is important in a clinical setting, as a patient with hypokalaemia will have a reduction in two of the three major repolarising cardiac currents which will lead to action potential prolongation as potentially being the trigger of arrhythmia. Another important regulator of the IK1 function is phosphatidylinositol 4,5-bisphosphate (PIP2) (Soom et al., 2001; Takano & Kuratomi, 2003). PIP2 is a quantitatively minor membrane component, although its local concentration may be relatively high. PIP is a key signalling phospholipid, whereby its hydrolysis by phospholipase C as well as its phosphorylation by PI3 kinases generates important second messengers. PIP2 binds directly to Kir channels, where it stabilises the open state. PIP2 has a high affinity with Kir2.X channels, which probably underlies the almost constitutive active IK1 (Lopes et al., 2002).
Summary
The length and morphology of cardiac action potential are shaped by the expression and fine-tuning of a number of ion channels. Sodium channels are responsible for the rapid depolarisation of the myocardium. The influx of sodium is followed by an influx of calcium through L-type calcium channels, contributing to keeping the depolarisation for several hundred milliseconds. The cardiac action potential is terminated by an increased efflux of potassium driving the membrane potential towards repolarisation. The dynamic properties of the action potential are obtained through a number of regulatory mechanisms maintaining the delicate balance between the different depolarising and repolarising ionic currents. Many of the primary regulatory mechanisms – such as P-subunits and phosphorylation sites – have been established. However, below the direct channel interacting proteins there is a whole network of modulatory mechanisms, and we are only just on the brink of discovering their role in regulating the cardiac action potential.