Human phenotypic studies
Locus-specific triggers
While QT interval prolongation puts patients at risk for abnormal heart rhythms, most patients are asymptomatic on a daily basis, with arrhythmias triggered by certain conditions or stimuli. In a 2001 study of 670 patients with known symptomatic LQT1, LQT2, or LQT3, a correlation between genotype and one of three specific triggers: exercise, emotion, or sleep was found. LQT1 patients had most events (syncope, cardiac arrest, or sudden death) triggered by exercise (62% of cases), while LQT2 patients had most events triggered by emotion (43% of cases), and LQT3 patients had most events during sleep (39% of cases) (Schwartz et al., 2001). In another study exercise induced significant further prolongation of QTc in LQT1 patients compared to LQT2 (Takenaka et al., 2003). In mice with an LQT3 knock-in mutation, bradycardia induced by cholinergic stimulation provoked torsade de pointes, while physical stress, mental stress, isoproterenol, and atropine did not (Fabritz et al., 2010). In female LQT2 patients, the post-partum period is a time of increased risk for arrhythmia (Khositseth et al., 2004). These efforts to categorize locus-specific triggers help clinicians in initial diagnostic phases and to better advise patients diagnosed with a specific LQTS genotype. There are some overlaps in triggers; for example, a certain percentage of LQT2 patients have cardiac events triggered by exercise. One study found a correlation of mutation location within HERG and the type of trigger causing symptoms: pore-loop mutations correlated with arousal-triggered events, non-pore mutations more often associated with exercise-triggered events (Kim et al., 2010).
Therapeutic approaches
Currently, there are five main avenues for treatment for adult patients with LQTS: (1) P-blockers, (2) gene-specific therapy, (3) pacemakers, (4) left cervico-thoracic sympathetic ganglionectomy, and (5) implanted cardio-verter defibrillators (ICDs). The primary goal of these therapies is to prevent life-threatening ventricular tachyarrhythmias and sudden cardiac death.
Given the correlation of LQTS locus and specific arrhythmia triggers, an important part of LQTS management is avoidance of triggers. LQT1 patients are advised to avoid competitive and endurance athletics, especially swimming. LQT2 patients are advised to reduce exposure to startle-stimuli, such as loud alarm clocks. LQT3 patients may have a pacemaker implanted to prevent bradycardia during sleep. For all patients with a LQTS diagnosis, the first line treatments demand avoidance of all potentially QT-prolonging drugs and the correction of electrolyte imbalances or other precipitating metabolic conditions. Pharmacological treatment may used in combination with trigger avoidance. P-blocker therapy is widely used for treatment of LQT1 and LQT2, having been associated with significant risk-reduction in adult and pediatric cases and is considered a treatment with very low risk of adverse effects (Goldenberg et al., 2010). Mortality of patients on P-blockers is around 0.5% (Schwartz, Priori et al., 2001; Priori et al., 2004). Channel blockers or openers may also be used, though they can be pro-arrhythmic if not properly monitored. A study examining the effects of the K+ channel opener nicorandil on canine models of LQT1, 2 and 3 showed that the drug may be effective in shortening the QT interval and preventing torsade de pointes in LQT1 and LQT2, but not LQT3 (Shimizu & Antzelevitch, 2000). For LQT3 patients where cardiac events are more likely to happen at low heart rates, P-blocker therapy is generally less helpful. LQT3 who have mutations in SCN5A where the defect is a persistent late current, channel blockers such as mexilitene, or flecainide are potentially helpful (Rosero et al., 1997).
Novel approaches include potassium supplementation for LQT2 patients. In vitro experiments have showed that proper intracellular K+ concentration is a requirement for normal HERG channel trafficking to the membrane, and that extracellular potassium modulates HERG current (Guo et al., 2009; Wang et al., 2009). These findings correlate with earlier studies that focused on HERG current density, showing that IKr current paradoxically increased when extracellular K+ concentration was increased (Sanguinetti & Jurkiewicz, 1992). One group administered spironolactone to eight LQT2 patients for four weeks, and observed a decrease in QT interval, (Etheridge et al., 2003) while another treated seven subjects with potassium supplementation and had similar findings (Compton et al., 1996). Such approaches may be considered in LQT2 patients.
Invasive therapies include left cardiac sympathetic denervation (LCSD), stellate ganglionectomy, and implantable cardioverter defibrillators (ICDs). LCSD involves the removal or ablation of the first four thoracic ganglia (which includes the stellate ganglion). In a 2004 study that included 174 high-risk, symptomatic LQTS patients who underwent LCSD, post-surgical QT intervals were shortened, and there was a 91% reduction in cardiac events over eight years of follow-up (Schwartz et al., 2004). These types of surgical interventions decrease sympathetic stimulation to the heart and may be recommended for patients who have not experienced cardiac arrest, but still experience syncope while on P-blocker therapy. ICD placement in such patients may be problematic because they may receive an intolerably high number of shocks. ICDs are most appropriate for patients who have already had an episode of cardiac arrest and are at higher risk for recurrence.
Male / female differences
To date, all LQTS loci are autosomal and not sex-linked. There are however, interesting differences between male and female LQTS patients. The QTc for women during the reproductive years (age 16-45) is longer than that for men of the same age (Bazett, 1920).
Women also have a higher resting heart rate than men (Ashman, 1942; Jose & Collison, 1970). The QTc intervals for males and females under age 16 are comparable as are those of post-menopausal women and men of the same age (Locati et al., 1998). There is also an increased risk for women of reproductive age with LQT1 and LQT2 mutations to have arrhythmic events (Zareba et al., 1995); (Lehmann et al., 1997). These findings implicate differential affects of the sex hormone pathways on cardiac electrophysiology. Interestingly, there is an increased risk of having a cardiac event for female LQT1 and LQT2 patients in the immediate post-partum period (Seth et al., 2007). Another recent report described a patient with KCNE1 mutation who experienced aborted sudden cardiac death in the post-partum period (Nakajima et al., 2010). The current recommendation is to continue P-blocker therapy throughout the pregnancy and post-partum period to avoid cardiac events. While LQT1 and LQT2 mutations seem to adversely affect women more, the LQT3 (and Brugada syndrome mutations) event rate is greater in men (Priori et al., 2003). Among LQT3 genotyped individuals, men have a longer QTc than women. Another important condition where there are significant male/female differences is in acquired LQTS that may occur with drugs that block K+ channels, mainly HERG. Multiple studies found that women are more likely to have adverse events when taking a QT-prolonging medication (Woosley & Sale, 1993; Drici et al., 1996; Reinoehl et al., 1996). This should be a key consideration when prescribing medications to patients with LQTS and in the general population.
New model systems: Induced patient-specific stem cells (iPSCs)
A novel model that has been under recent investigation to better understand the pathophysiology of LQTS is induced patient-specific stem cells. This process consists of obtaining skin cells (dermal fibroblasts) from patients with known LQTS mutations as well as unaffected control subjects, culturing them, de-differentiating them into pluripotent stem cells, and re-differentiating them into cardiomyocytes in vitro. The dermal fibroblasts are infected with retroviruses or lentiviruses containing specific transcription factors that convert and reprogram the dermal cell to a pluripotent stem cell without affecting the other genomic DNA containing the LQTS mutation. The pluripotent stem cells are then given specific growth factors in a precise order and grown on feeder cells until they form embryoid bodies: aggregates of cells that can differentiate into cardiomyocytes of three distinct types: "nodal", "atrial" and "ventricular" (Zhang et al., 2009). The cells were also shown to have cardiomyocyte architecture including sarcomeric organization of actin, myosin and other components, albeit immature.
In 2010, Moretti et al. characterized cells derived from a LQT1 patient who had the mutation R190Q in KCNQ1. They showed that these cells exhibited a prolonged APD due to reduced IKs current density (Moretti et al., 2010). Itzhaki et al. derived cardiomyocytes from a patient with an LQT2 mutation in HERG (A614V); these cells also showed a prolonged APD and reduction in IKr (Itzhaki et al., 2011). They used microelectrode arrays to record from groups of mutant cells and showed an increased incidence of EADs. To study mutations in the calcium channel, the group of Yazawa and colleagues were able to derive iPSCs from patients with LQT8 (Timothy syndrome) (Yazawa et al., 2011). They found that the mutation-carrying cells contracted slowly and irregularly, had exaggerated calcium influx with prolonged APD in ventricular type cells. These studies were able to confirm previous findings of channel dysfunction in a more native setting.
Two notable caveats with the iPSC approach are that the differentiated cells are immature and may not express the full complement of ion channels and accessory or regulatory proteins and cellular architecture as does an adult cardiomyocyte and that the differentiated cells may be heterogeneous. A challenge is to develop a selection method or purification scheme to isolate the induced cardiomyocytes in larger and more uniform quantities. The iPSC system holds particular promise in determining the effect of potentially deleterious mutations in proteins other than ion channels such as regulatory or scaffolding proteins. This system may also be of particular utility in analysis of mutations non-coding areas (introns promoters, splice-sites and untranslated mRNA sequences). For therapeutics, iPSCs may provide a platform to test new potential pharmacologic approaches in a more native and genotype-specific setting prior to testing in animals and humans.
Mechanisms of deleterious mutations
LQTS mutations cause alterations in cardiac ionic currents that result in delayed action potential repolarization. The delay can be caused by sustained inward sodium or calcium currents, or impaired outward K+ current. Mutations to ion channels or their regulatory proteins alter channel function such that an increase or decrease in current occurs; the mechanism by which the mutation causes these functional changes can be categorized into several classes: (1) changes in biophysical properties, (2) changes in channel synthesis and processing, and (3) changes channel regulation.
Biophysical
Biophysical defects are caused by mutation to channel subunits that result in channel gain or loss of function. Several biophysical parameters affect how much current a channel carries: the structure of the channel pore, channel gating, and the stability of the channel in the open versus closed states.
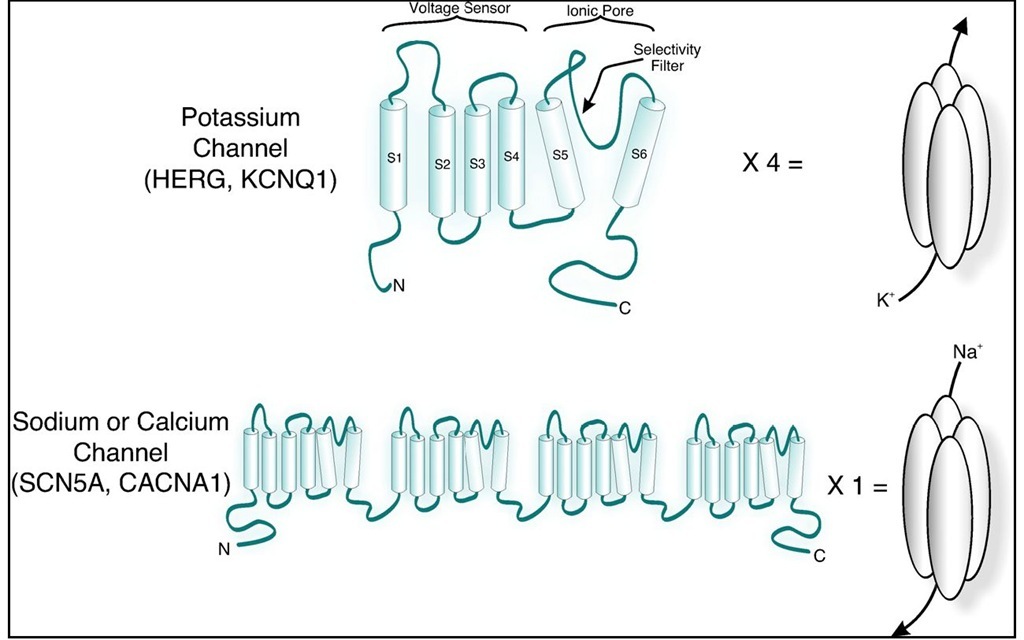
To discuss the effects on channel structure, we will focus on voltage-gated K+ channels as an example (see Figure 1). The pore of a K+ channel subunit is composed of two transmembrane helices (S5 and S6) and an intervening loop; when tetramerized, the loops form the K+ selectivity filter that extends into the ion conduction pathway, while the helices line the remainder of the pore (Doyle, Morais Cabral et al., 1998; Jiang, Lee et al., 2002; Jiang, Lee et al., 2003; Long, Campbell et al., 2005). The structure of the selectivity filter is rigid as ion selectivity is based on size; it holds the same conformation regardless of whether the channel is open or closed. The pore-lining transmembrane helices though, move in response to changes in membrane voltage; when the channel is closed, the intracellular end of the helices prevent ions from accessing the selectivity filter, and when the channel is open, the helices are positioned such that ions can enter the pore. Deleterious mutations have been identified in the pore region. They presumably alter the structure of this sensitive region such that ions cannot access the selectivity filter, or cannot pass through the selectivity filter. A second region that may be affected is the voltage sensor. The S4 transmembrane domain of a K+ channel is lined with positively charged amino acids. A change in the membrane potential causes movement in the voltage-sensor, and subsequently the pore region to which it is linked. Mutation to the voltage-sensing domain can result in delayed or impaired channel opening. Analysis of several LQT1 mutations in the S4 domain revealed a depolarizing shift in voltage-dependent activation of the channel, which indicates that a larger driving force was required to open the mutant channels (Henrion et al., 2009). Though S4 contains the voltage-sensing residues, transmembrane domains S1 to S4 are structurally clustered together as the voltage-sensing domain; thus, mutations to residues in S1, S2, and S3 have also been associated with LQTS.
Unlike in K+ channels, where loss-of-function mutations are the pathophysiologial defect, in Na+ channels, gain-of-function mutations lead to an increased Na+ current that maintain the cell in a depolarized state. Na+ channels are responsible for the rapid influx of Na+ ions in phase 0 of the action potential; this phase is extremely short-lived (milliseconds) as Na+ channels normally rapidly inactivate. Mutations that alter Na+ channel inactivation (rather than activation or deactivation) account for the majority of LQT3 . A defect in inactivation leads to a persistent Na+ current throughout the action potential duration, which delays repolarization. Several cytoplasmic regions of the Na+ channel are responsible for inactivation, and mutations in these regions lead to persistent current (Jones & Ruben, 2008). Biophysical mutations can act in a dominant-negative manner in patients with one wild-type allele and one mutant allele. Because K+ channels are composed of four separate, identical channel subunits, wild-type and mutant subunits randomly combine together. Mutations that act in a dominant-negative manner may affect the function of channels that contain even one mutant subunit; less severe mutations may result in heteromeric channels with normal function or a partial defect. Sodium and calcium channels, however, are encoded such that the entire pore-forming channel is translated into a single polypeptide. Therefore, a patient who inherits a single mutant allele will have roughly 50% normal and 50% mutant channels. The mechanisms for dominant phenotype in these cases resides in the fact that LQTS mutations in sodium and calcium channels are "gain-of-function" which cannot be overcome by expression of the normal allele.
Fig. 1. Schematic representation of K+ (top) and sodium or calcium (bottom) channel proteins. Note that four identical subunits combine to form a single unit for K+ channels. Sodium and calcium channels are encoded as a singe polypeptide that is compised of four domains that are homologous the single subunit of a K+ channel. S1 through S6 signify the transmembrane helices
Cell biological processes
Errors in synthesis
The first steps in channel synthesis include transcription of RNA in the nucleus and post-transcriptional modifications (capping, addition of a poly-A tail, splicing, and editing). The mature mRNA is then targeted to ribosomes on the endoplasmic reticulum to begin protein translation. Several classes of mutations can change mRNA stability and negatively affect abundance of functional protein. These include frame-shift and premature termination codons. These types of mutations may cause mRNA instability and subsequent degradation, a process called nonsense-mediated decay (NMD). NMD has been shown to be an underlying mechanism in other diseases. This phenomenon has been implicated as a cause recurrent intrauterine fetal death or LQTS in mutations of HERG (Bhuiyan et al., 2008) (Zarraga et al., 2011). Similarly NMD has been implicated to play a role in LQT3 mutation of the Na+ channel SCN5A (Teng et al., 2009). It is certainly conceivable that mutations, yet to be described, may also introduce new binding motifs for micro-RNAs that would alter stability and the mRNA and hence, protein synthesis.
Errors in trafficking
In general, defective protein trafficking is emerging as an important disease mechanism that concerns a variety of cell types. A newly synthesized channel goes through numerous processing steps before it ultimately reaches the membrane and is functional. At the earliest stage, some signaling systems may affect channel synthesis itself (Chen et al., 2009; Chen et al., 2010; Sroubek & McDonald, 2011). After the channel is synthesized at the endoplasmic reticulum (ER) it must fold to attain its tertiary structure and then assemble with other subunits to form the functional macromolecular complex. Folding is a complex process involving helper proteins called chaperones, which work in iterations to achieve the final proper conformation. Once the protein is properly folded it may be glycosylated and it leaves the ER through vesicle transport to arrive at the Golgi. At the Golgi the glycoslyations may be further modified and finally the channel leaves the Golgi in vesicles bound for the plasma membrane.
Mutations may cause channel proteins to fold improperly; these mis-folded proteins may be recognized by the quality-control system and marked for degradation by the proteasome. This causes a trafficking error, and mis-folded protein may accumulate in the ER or Golgi membrane. Though severe mis-folding results in a non-functional channel (for example, mutations that prevent tetramerization of channel subunits), milder mutations may allow for a functional channel to fold, yet still be retained intracellularly. This is in theory possible, but under most circumstances it is difficult to test because functional experiments such as electrophysiology require proper trafficking. Mutations in HERG and KCNQ1 that affect trafficking can be loss-of-function and many of them can act in a dominant-negative fashion interfering with associated normal allele subunits. While tetramerization has been studied for these channels, the mechanisms are incompletely understood. Given a situation where wild-type and mutant subunits are co-expressed, the heterogeneous pool of tetrameric channels may express a range of current density from zero to the full wild-type amount. This could explain why some trafficking mutations have a more severe phenotype than others.
It is worth considering LQTS mutation-associated trafficking errors in HERG. A trafficking defect is the most common cellular phenotype for LQT2 mutants (Anderson et al., 2006). Particular attention has been paid to the HERG cytoplasmic C-terminal portion where analysis of various LQT2 mutations has revealed that this segment is critical for tetrameric assembly and proper trafficking. While many of these studies have focused on the C-terminus of HERG, it is important to note that trafficking defective mutants have also been found throughout the N-terminus as well as the transmembrane domains (Balijepalli et al., 2010). Complex mechanisms for the forward trafficking (from ER to Golgi) of HERG have been suggested. Recently, Delisle et al. showed that HEK cell expressed HERG undergoes COPII-dependent ER export and also endosomal trafficking which determine its plasma membrane expression (Delisle et al., 2009). They also showed that this atypical trafficking route is mediated by small GTPases such as Sar1 and Rab11b. More recent trafficking studies show that LQT2 mutants may be subjected to quality-control in the ER-Golgi intermediate compartment (ERGIC) (Smith et al., 2011). It has also been shown that trafficking defective LQT2 mutants are subsequently degraded by the ER-associated degradation pathway (ERAD) and the ubiquitin proteasome pathway (Kagan et al., 2000; Gong et al., 2005). While this picture is incomplete (studies rely on heterologous expression), it does give us insight into the points during synthesis where HERG is particularly susceptible and how mutations affect its maturation.
Recent studies have examined the role of extracellular potassium in the endocytosis and degradation of HERG. Recently, the work of Guo and colleagues has provided a biochemical basis and mechanistic approach to study the behavior of HERG in low-potassium conditions. The 155 kDa form of HERG undergoes endocytic internalization from the plasma membrane and proteasomal degradation through a mechanism involving caveolin (Massaeli et al., 2010). Further work was done by Massaeli and colleagues who studied the behavior of pore-lining mutations in HERG under zero-potassium conditions. They found that alanine mutants at certain positions in the pore helix and selectivity filters abolished the low-potassium induced degradation. This is an interesting mechanism since arrhythmias are often precipitated by electrolyte disturbances such as hypokalemia (Berthet et al., 1999).