17.2.
Mechanism of Pollutant Formation
Petrol is a complex blend of hydrocarbons such as paraffins, cycloparaffins, olefins and aromatics, which contain mainly carbon and hydrogen atoms. In an automobile engine these hydrocarbon compounds are burnt with air to produce energy for propelling the vehicle.
Burning is the chemical process of oxidization, in which the hydrocarbons are combined with the oxygen in air. If sufficient oxygen is available, the hydrocarbon is completely oxidized so that all of the carbon atoms combine with oxygen to produce CO2 (carbon dioxide) and all of the hydrogen atoms combine with oxygen to produce H2O (water), and both of these substances are harmless.
![]()
For stoichiometric (chemically perfect) combustion an air-fuel ratio of about 14.7 : 1 is required. This means for completely burning of 1 gm of petrol, consisting mainly of heptane (H7H16) and hexane (C6H14), about 14.7 gm of air are required (a volume of about 12.2 litres). In practice, air-fuel mixtures may be either fuel-rich or fuel-lean. Therefore, the ratio of the actual air-fuel ratio to the stoichiometric air-fuel ratio is considered as a useful parameter for describing mixture strength. This parameter, termed as relative air-fuel ratio or excess air factor, is denoted by the symbol X (the Greek letter lambda) and is defined as,

Complete oxidization rarely takes place in a spark-ignition engine even with a stoichiometric mixture, because the hydrocarbons are forced to burn in a short period of time with a fixed volume of air. Atmospheric nitrogen, which is normally chemically inert, starts to react with oxygen at high temperature and pressure prevailing in the combustion chamber. The nitrogen thus consumes oxygen, which otherwise react with the hydrocarbons, leading to their incomplete combustion. This resulted in the formation of undesirable combustion products, specifically the pollutants CO (carbon monoxide), HC (unburned hydrocarbon) and various oxides of nitrogen (NO, NO2 and N2O ; collectively termed NOx), i.e.
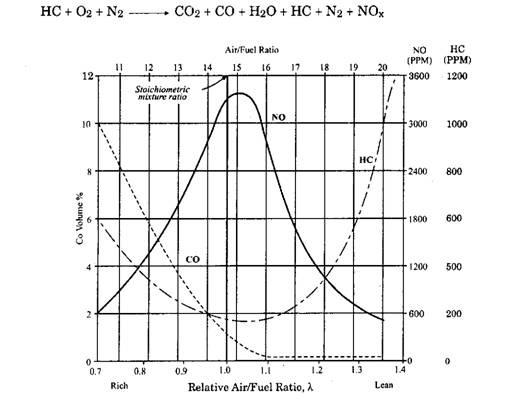
Fig. 17.2. Pollutant emission as a function of relative air-fuel ratio.
Even with a stoichiometric mixture (X = 1) pollutant gases (HC, CO and NOx) are produced, which constitute up to 2 percent of the exhaust gas. The relative quantities of the pollutants produced by the engine depend principally upon the relative air-fuel mixture ratio, and vary in the manner illustrated in Fig. 17.2. Maximum combustion temperature occurs at slightly fuel lean mixture of about X = 1.05 (an air-fuel ratio of about 15.5 : 1) and decreases rapidly to either side. Since NOx emission are temperature dependent they tend to follow this characteristic and so decrease rapidly as X increases.
On the other hand HC emissions increase away from stoichiometric. On the rich side (X < 1) this arises as an out come of incomplete combustion due to an inadequate oxygen supply. On the lean side (X > 1) slow or incomplete burning causes the exhaust valve to open before combustion is entirely completed, allowing some unburned HC into the atmosphere. With very lean mixtures, misfire may occur so that HC emissions rise sharply.
