Phyllocarida
(Leptostracans)
Subphylum Arthropoda
Class Malacostraca
Number of families 3
Thumbnail description
Small crustaceans with a laterally flattened carapace enclosing the bases of the thoracopods (legs), a hinged rostrum covering stalked eyes, and a tapering abdomen ending in a forked tail
Illustration: Levinebelia maria.

Evolution and systematics
Only one fossil leptostracan has been recognized in the literature, the Permian Rhabdouraea bentzi. It has a separate family status (Rhabdouraeidae; Schram and Malzahn, 1984). However, the fossil is only that of an abdomen and makes comparison to other leptostracans difficult. The regular segmentation of the body, the trunk musculature, nervous system, circulatory system, presence of a furca, and absence of uropods indicate that the Leptostraca are primitive malacos-tracans. Three extant families are recognized: Nebaliidae (with genera Nebalia, Nebaliella, Dahlella, and Speonebalia); Nebaliopsidae (with only one genus, Nebaliopsis, and only one species, N. typica); and Paranebaliidae, containing genera Paranebalia and Levinebalia. Almost 35 species are described. There is not a common name for the leptostracans, but names such as “leaflike-shrimps,” “mud-shrimps,” and “sea fleas,” are used in the literature.
Physical characteristics
Leptostracans have a bivalved carapace (the two valves are fused dorsomedially but have no hinge) enclosing head and thorax, or enclosing head, thorax, and part of the abdomen, laterally compressed and smooth. The anterior end of the carapace dorsum continues as a movable articulated rostrum. The eyes are stalked, compound, with visual elements either present or absent (genus Dahlella). The antennules (antenna one) are usually biramous and the antennae (antenna two) are uni-ramous. The eight pairs of thoracic limbs (thoracopods) are leaflike turgor appendages, foliaceous (phyllopodous), and bi-ramous. The abdomen has six pairs of appendages (pleopods): the first four pairs are birramous and large, and the last two are uniramous and reduced. The seventh abdominal segment continues as a telson having a long caudal furca.
Most leptostracans are 0.19-0.59 in (5-15 mm) long, but one species (Nebaliopsis typica) is a giant at nearly 1.5 in (4 cm) in length.
In life, specimens appeared mostly transparent (colorless), except for the bright red eyes. When viewed with the naked eye the thoracic region appears almost white. All leptostra-cans, except Nebaliopsis typica, show pronounced sexual dimorphism. Males also occur much more rarely than females. The carapace of the male is less deep than that of the female and the antennae are much longer.
Distribution
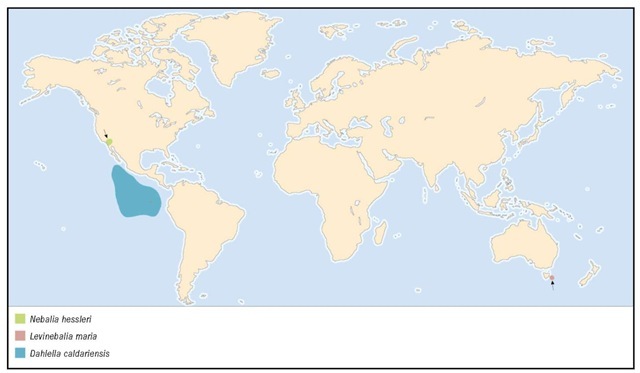
The genera of Leptostraca are distributed differently. Nebalia is cosmopolitan. Nebaliella is confined to cold waters, being found in Antarctica, southern Australia, and the high latitudes of the Northern Hemisphere. Nebaliopsis is a pelagic genus with a worldwide distribution. Paranebalia is found in central America, Bermuda, New Caledonia, and Australia. Levinebalia has been recorded only in Australia and New Zealand. Dahlella was collected from hydrothermal vents near the Galapagos, and Speonebalia has been recorded from only marine caves in the Turks and Caicos Islands.
Habitat
All leptostracans are marine, and have been recorded in waters from 3.2 ft (1 m) deep to more than 6,561 ft (2,000 m); most species occur in <656 ft (<200 m). Many leptostra-cans, with the exception of the pelagic Nebaliopsis typica, seem to prefer mud bottoms that are low in oxygen content. However, leptostracans can be found in a variety of habitats ranging from the intertidal zone to the abyssal deeps.
Behavior
Young animals and females rest in the same place for hours, their thoracic limbs beating rhythmically, moving respiratory currents of water through the carapace. The mature males may swim long distances propelled by the four anterior pairs of pleopods. Each of these pleopods is hooked to its partner so that each pair functions as a unit when swimming.
When placed in laboratory tanks or small, concave “dishes” of glassware (watch glasses) with mud, leptostracans will swim right to the bottom and burrow in. Frequently, not one part of the body is moved at all, and even the heartbeat slows down; these are functional and physiological adaptations to living in low-oxygen environments.
Feeding ecology and diet
Living in muddy environments, often where organic matter is abundant, it is assumed that leptostracans are filter feeders, but they are often taken in baited traps, which indicates that they act as at least opportunistic scavengers.
Most of the benthic leptostracans suspension feed by stirring up bottom sediments. They are also capable of grasping relatively large bits of food (detritus particles, dead animals) directly with the mandibles.
Reproductive biology
The eggs of all leptostracans, except possibly in Nebaliop-sis, are carried by the female in the thoracopod brood cham-
ber beneath her carapace. Development is embryonic and the young hatch as post larvae. These post-larval, or mancoid, stages differ from the adults in having a rudimentary fourth pleopod. The free-swimming, pelagic larva of Nebaliopsis has been found in plankton collections. In this genus, the eggs may not be carried in a brood pouch but laid directly into the water.
Water temperatures influence the length of time taken to reach maturity, the size at maturity, and the incubation time of young.
Transformations in the shape of the carapace, antennae, pleopods, and furca are gradual from molt to molt in immature and subadult males, and deviate from the female morphology, which generally remains unchanged except when reproductive.
Conservation status
Nothing is known. No species are listed by the IUCN.
Significance to humans
Nebalia bipes is considered to be desirable as a living fish food and for larval rearing because of its suitable size and tolerance to the deterioration of bottom conditions.

1. Levinebalia maria; 2. Nebalia hessleri; 3. Dahlella caldariensis.
Species accounts
No common name
Dahlella caldariensis
ORDER
Leptostraca
FAMILY
Nebaliidae
TAXONOMY
Dahlella caldariensis Hessler, 1984, Galapagos.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
Body approximately 0.31 in (8.1 mm) long (base of rostrum to tip of telson of largest individual); carapace 0.23 in (6 mm) long, ovoid, deepest one-third from front, 1.3-1.5 times longer than deep; posterior edge emarginate, exposing at least part of pleonite one dorsally. Rostrum 0.4 carapace length, three times longer than wide, without keel (ventral projection), but with proximal midventral device that loosely interlocks with eye structures. Eyestalk strongly curved (banana shape), surface with denticles; live at deep-sea hydrothermal vents, without visual surface (pigmentation).
DISTRIBUTION
Common at hydrothermal vents on the Galapagos, spreading center and the East Pacific Rise at 13°N and 21°N;
8,040-8,595 ft (2,450-2,620 m) depth.
HABITAT
Found in the throat of vent openings and clumps of mussels and vestimentiferans (“beard worms”).
BEHAVIOR
Nothing is known.
FEEDING ECOLOGY AND DIET
Hessler (1984) suggested the toothed anterior edge of the eye-stalk (pointed laterally beyond the carapace) may be used to scrape surfaces to loosen food such as bacterial encrustations.
REPRODUCTIVE BIOLOGY
Smallest individuals at all localities (0.035 in [0.9 mm] from base of rostrum to tip of telson) were considered by Hessler (1984) the first free-living instar, called mancoid stage.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
No common name
Nebalia hessleri
ORDER
Leptostraca
FAMILY
Nebaliidae
TAXONOMY
Nebalia hessleri Martin Vetter, and Cash-Clark, 1996, Scripps Canyon, La Jolla, San Diego, California, United States.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
Total length (excluding setation) up to 0.59 in (15 mm) (average total length 0.38 in [9.8 mm]). Carapace length of mature female is up to 0.26 in (6.8 mm), approximately 1.5 times longer than deep, unsculptured, with usual posterodorsal indentation. Rostrum long, clearly extending beyond eye, distally rounded, length approximately 2.7 times width; with ventral projection or “keel” more or less rectangular, unpaired, but with slight medial depression gently sloping upward toward ventral surface of rostrum and with slightly protruding, blunt anterolateral corners. Compound eye is large, well developed; pigmented (visual surface), extensive, covering at least distal half. Telson short, approximately as long as wide, rectangular, or with sides slightly diverging posteriorly. Caudal furcae elongate, approximately twice length of telson, and sometimes greater than twice its length;; acute spines along posterior dorsal borders of pleonites. Males have second antenna that is curved rather sharply in an anterior direction, but considerable variation exists (from extending almost directly anteriorly up to appearing slightly cork-screwed).
DISTRIBUTION
To date, known only from the type locality.
HABITAT
Detrital mats with low oxygen levels in submarine canyons off the coast of southern California at depths of 60 ft (19 m).
BEHAVIOR
Nothing is known.
FEEDING ECOLOGY AND DIET
Within the detritus mat habitat of these animals, vertebrate and invertebrate carcasses are frequently seen and exploited by Nebalia hessleri. In the laboratory, this leptostracan feeds on large pieces of meat (e.g., fish, shrimp, fish-containing cat food, and squid) by scraping off chunks, using the peduncle of the first antenna, which bears a row of strong spines. The animals often remained in contact with the meat for five minutes or more, and produced a continuous fecal strand that sometimes rivaled the length of the animal. In such cases, the fecal strand was the same color as the food and thus possibly was poorly processed.
REPRODUCTIVE BIOLOGY
Eggs, brooded under the carapace of the female, are gold or cream colored.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
None known.
No common name
Levinebalia maria
ORDER
Leptostraca
FAMILY
Paranebaliidae
TAXONOMY
Levinebalia maria Walker-Smith, 2000, Tasman Sea.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
Female entire length 0.18 in (4.8 mm). Carapace length 0.11 in (3 mm), depth 0.07 in (2 mm), emarginate, dorsally convex, anterior and posterior margin rounded, 3.6 times length of rostrum, posterior margin reaching pleonite four, surface not sculptured. Rostrum length 2.8 times width, greatest depth 0.2 times length, subterminal spine present, keel absent. Eyestalks pigmented, dorsal margin slightly convex, without denticles or cuticular outgrowths. Caudal furca 2.4 times long as wide, 1.5 times as long as telson. Juvenile male (fully mature male has not been found) entire length 0.17 in (4.4 mm).
DISTRIBUTION
Tasman Sea, 9.3 mi (15 km) E of Maria Island, Tasmania, Australia, at 334 ft (102 m) depth.
HABITAT
Epibenthic, in relation with fine muddy bryozoan sand and coarse, shelly sand. In waters from 328 to 656 ft (100-200 m) deep.
BEHAVIOR
Nothing is known.
FEEDING ECOLOGY AND DIET
Nothing is known.
REPRODUCTIVE BIOLOGY
Nothing is known.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
None known.