Cephalopoda
(Nautilids, octopods, cuttlefishes, squids, and relatives)
Phylum Mollusca
Number of families About 45
Thumbnail description
Mollusks bearing a radula (toothed tongue) and well-developed heads; mouth characterized by a dorsoventral pair of horny jaws known as beaks and encircled by the bases of 8-ca. 60 grasping appendages; single pair of lateral, image-forming eyes; well-developed brain and peripheral nervous system
Photo: A greater blue ringed octopus (Hapalo-chlaena lunulata) takes a crab as it emerges from its hole.

Evolution and systematics
Cephalopod shells are very well represented in the fossil record as far back as the Upper Cambrian period, about 505 million years ago. Soft-tissue fossils, however, are extremely rare. Two very different subclasses of cephalopods live in modern seas: (1) the nautilids (subclass Nautiloidea), represented by two genera and about six species that have two pairs of gills and external shells into which they can withdraw; and (2) the neocoleoids (a division of subclass Coleoidea), sometimes called dibranchiates because they have a single pair of gills, comprising all other living species of cephalopods (fewer than a thousand). The neocoleoids include the familiar squids, cuttlefishes, and octopods.
There are about 44 families of extant neocoleoids, plus one family of nautilids. Only eight families have more than 20 species; many families consist of only one species or one genus. Although the familial relationships of living cephalopods are fairly stable, researchers have not completely resolved many relationships at higher and lower levels of classification (orders and genera). Eight distinctive groups of neocoleoids can be defined, however: Incirrata (common octopods); Cirrata (finned octopods); Vampyromorpha (the vampire squid, one species); Sepiida (cuttlefishes), Spirulida (the ram’s horn squid, one species); Sepiolida (bobtail and bottle squids); Myopsida
(inshore squids), and Oegopsida (oceanic squids). There is no consensus regarding the taxonomic level of these groups, and some families cannot be assigned with confidence to any of them. The first three groups listed comprise the Octopodi-formes; the remaining five are grouped together as the De-capodiformes, commonly called decapods.
Physical characteristics
With regard to general structure, cephalopods have three easily distinguished regions. From front to rear, these regions are: (1) the brachial crown (arms and tentacles) surrounding the mouth; (2) the head, with prominent lateral eyes; and (3) the mantle, which may have a pair of fins on the sides. This overall three-part structure is less distinct in nautilids but is still recognizable. Nautilids have approximately 60 arms (sometimes called tentacles or cirri) arranged in two rings around the mouth. Neocoleoids have a single ring of either eight or ten appendages surrounding the mouth. On those that have ten, two appendages are modified into either ven-trolateral tentacles (decapods) or dorsolateral velar filaments (vampires). Thus, all living cephalopods other than the nau-tilids have eight arms, and some have either two additional tentacles or two filaments. Although some nonspecialists refer to all cephalopod appendages as tentacles, one should
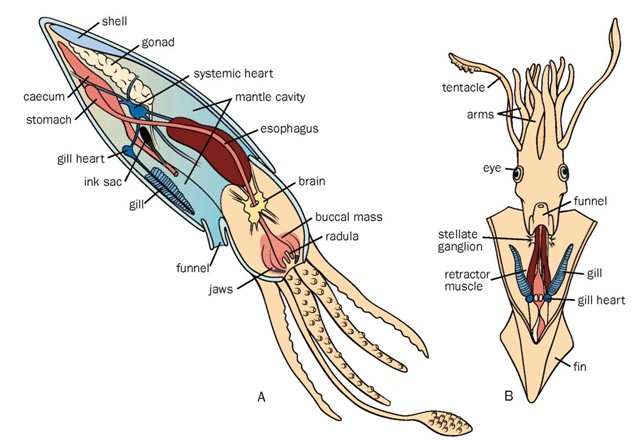
Cephalopod anatomy. A. Lateral view; B. Ventral view.
avoid this usage because it confuses the differentiation of the specialized appendages in decapods and vampire squids.
Cephalopods range in size from the giant squids, Archi-teuthis spp., which are commonly longer than 6.56 ft (2 m) in mantle length (ML) and reportedly reach 16.4 ft (5 m) in ML, 59 ft (18 m) in total length, and 661.3 pounds (300 kg) in weight, to tiny species like Idiosepius spp., decapods which mature at an ML of about 0.23-0.31 in (6-8 mm); or the sexually dimorphic pelagic octopods, Argonauta spp., in which mature males are only 0.39 in (1 cm) in ML. Several squid species and at least two species of octopods reach sizes larger than an adult human. They include the true giant squid as well as the muscular (and dangerous) ommastrephids, the weakly muscled cranchiids and chiroteuthids, and the giant Pacific octopus or “devilfish.”
Many species of squids, cuttlefishes, and octopods can radically change their appearance within a fraction of a second. This ability to transform themselves so rapidly is the reason that cephalopods have been called masters of disguise. These remarkable transformations result from interactions among brown, red and yellow pigment-filled chromatophore (color-producing) organs under the control of the animal’s nervous system; reflective iridophores (cells that produce a silvery or iridescent pigment); fixed white leucophores (pigment cells containing guanine); fixed tubercles (small nodules); and erectile muscular flaps and papillae in the animal’s skin. Furthermore, some cephalopods have light-producing organs called photophores. Cephalopod photophores come in two fundamentally different types. In the first type, known as intrinsic photophores, the light is produced biochemically by the squid or octopod. The second type, called bacterial photophores, makes use of symbiotic photogenic bacteria that grow in special chambers associated with the ink sac in the host. The light produced by both types of photophores is usually blue-green in color; the color can be altered, however, by structures associated with the photophore.
Other features include an ink gland and ink sac associated with the intestine that is characteristic of neocoleoids. Complex, highly developed nervous and sensory systems are also typical of living cephalopods, although less so for the nau-tilids than for the others. Especially noteworthy are the image-forming eyes, with lenses in the neocoleoids; and the complex brain developed from the nerve ring surrounding the esophagus. Other noteworthy peculiarities, including a muscular hydrostatic skeleton and a pattern of muscle fiber alignment known as oblique striation, also are characteristics of cephalopods.
Distribution
All cephalopods are limited to marine environments. Only a few species can tolerate the low levels of salt in the waters of estuaries and fjords, the minimum being 17.5 PSU (practical salinity units), or about half the saltiness of full-strength seawater. No cephalopods can live in freshwater. Some oc-topods are known to crawl from one tidal pool to another but cannot remain out of the water for long.
Habitat
All cephalopods are mobile throughout their lives, except for the egg stages that in many species are attached to various substrates. As far as we know, all cephalopods are either carnivorous (the neocoleoids) or scavengers (the nautilids) throughout their life cycles after hatching. Some researchers have proposed that some cephalopod paralarvae feed on phy-toplankton, but the evidence in support of this hypothesis currently is not very strong.
The life cycles of most neocoleoid cephalopods are very different from that of Nautilus. Whereas the latter is long-lived—it may live for 20 years or more—the lifestyles of other cephalopods have been characterized as “live fast and die young.” Their life spans seem to range from a few months for small species to a few years for larger species. Many of the generalizations that have been made for neocoleoid cepha-lopods, however, have been based on observations of a few coastal species whose habitats are convenient for research.
Some cephalopod species, especially squids, can be very abundant; they are occasionally among the dominant organisms in their ecosystems. Because they are important food for larger animals, in addition to being voracious predators, such species are key members of some marine food webs. There are, however, significant gaps in current information about the life history and ecology of these organisms. Furthermore, many generalizations about cephalopods based on one or a few species are turning out to be either questionable or wrong. For example, because the common European octopus, Octopus vulgaris, and the California market squid, Loligo opalescens, spawn once and then die, their reproductive cycle has been widely regarded as the general pattern for cephalopods. Researchers are accumulating evidence, however, that many species of squids and octopods spawn many times and continue to live and feed after laying their eggs. Squids have been thought not to care for their eggs because the coastal species that have been most closely observed, as well as a few oceanic species, seem to release their egg masses and then depart either by moving away or by dying. Recently, though, some gonatid squids have been found to carry their egg masses around after releasing them. We know so little about most cephalopod species, especially oceanic and deep-sea species, that many such surprises likely await discovery. In short, many generalizations about the group may need to be modified.
Cephalopods live in a range of water depths from inter-tidal levels to over 16,400 ft (5,000 m). Different marine ecosystems have very different cephalopod faunas. For example, no cuttlefishes are found in American waters. Many oc-topod species have recently been described from Antarctic Ocean waters, whereas the Arctic appears to have few octo-pod species. The deep sea is home to both primitive vampire squids and morphologically similar finned octopods as well as some strange oegopsid squids. Oegopsid squids tend to be dominant in epipelagic and mesopelagic oceanic waters (the upper layer of water that admits enough light for photosynthesis to occur, and the “twilight zone” just below it); whereas myopsid squids share the continental shelves with incirrate octopods and cuttlefishes. Some incirrate octopods are found in deep-sea habitats on the ocean bottom whereas others are entirely pelagic. Because cephalopods are so widespread throughout marine habitats, their patterns of habitat utilization also vary widely.
Behavior
Cephalopods are renowned for their large brains, well-developed eyes, and complex behavior. Their social organization varies from the solitary life of octopods through small schools of cuttlefish to very large shoals of oceanic oegopsids. Some species of cuttlefishes and squids are known to form seasonal aggregations or gatherings for mating and spawning.
The sexes are separate in cephalopods. Courtship patterns vary from simple male grasping of the female followed by the implanting of spermatophores to complex courtship rituals in which both individuals display elaborate forms of touching prior to mating. Several species produce small “sneaker males” that resemble females; these sneaker males have been shown to be an important alternative to large, dominant mate-guarding males. The mating patterns of most oceanic cephalopods are largely unknown.
In addition to social organization and mating behavior, the use of visual communication among cephalopods has been a subject of debate. Whereas most observers who have watched cephalopods will agree that they perform complex visual signaling, these researchers debate whether such signaling can be considered a language. Cephalopod visual signaling involves a variety of behaviors, some of which are regularly directed toward conspecifics (e.g., mates or potential rivals), and others toward members of other species (e.g., prey or potential predators). The use of ink or other chemicals as an alarm signal to warn conspecifics has been demonstrated but not thoroughly investigated.
As with so many subjects about cephalopods, display patterns have been studied in detail for only a few easily accessible species. In general, these patterns include both acute patterns, lasting for a few seconds to a few minutes; and chronic patterns, lasting several minutes to several hours. The display patterns comprise chromatic (both dark and light), textural, postural, and movement changes. Commonly recorded displays include crypsis (hiding), in addition to what Hanlon and Messenger (1996) refer to as deimatic (threatening, startling, frightening, or bluffing) behaviors and protean (unpredictable or erratic) escape maneuvers.

A squid holds a fish in its tentacles.
Although some nearshore benthic octopods are known to occupy and defend territories for limited time periods, cephalopods generally are not territorial.
The primary variable in the activity patterns of cephalopods is the 24-hour diel cycle. Some neritic (coastal zone) species forage primarily by daylight, but most are nocturnal. Some species are crepuscular, which means that they are most active at dawn and dusk. Diel activities of oceanic species primarily involve vertical migration. The activity patterns of most deep-sea benthic species are unknown.
Many species of oceanic cephalopods undergo diel vertical migrations, wherein they live at depths of about 1,310-3,280 ft (400-1,000 m) during the day and then ascend into the uppermost 656 ft (200 m) of water during the night. Shifts in distribution over the course of a cephalopod’s life history are common. Some oegopsid squids are believed to undertake life-cycle migrations over large geographic distances covering hundreds of miles.
Feeding ecology and diet
Neocoleoid cephalopods are active predators that feed upon shrimps, crabs, fishes, other cephalopods, planktonic crustaceans, and, in the case of octopods, on other mollusks. Nautilids are scavengers.
Foraging behavior varies widely among species in various taxonomic groups. Some examples include rapid raptorial attacks (oegopsid and myopsid squids); raptorial attacks after stalking (cuttlefishes); drifting with dangling tentacles (some oegopsid squids); ambush from a hiding place (some bobtail squids, cuttlefishes, and benthic incirrate octopods); and perhaps even the use of mucus to entangle small prey (a cirrate octopod). Special foraging adaptations also vary widely among cephalopod species.
Cephalopods are major food items in the diets of many marine vertebrates, including toothed whales; seals; pelagic birds (penguins, petrels, albatrosses, etc.); and both benthic and pelagic fishes (e.g., sea basses, lancetfishes, tunas, bill-fishes).
Reproductive biology
The sexes are separate in all cephalopods. The unpaired gonads, either ovary or testis, are always located in the posterior region of the mantle cavity. Females may have one or two oviducts depending on their major taxonomic group. Each oviduct has an oviducal gland that secretes a primary coating around the egg. Many female cephalopods have an additional nidimental gland that adds extra layers of protective coating to the egg. This gland is found beyond the end of the oviduct.
In males, the sperm passes through the spermatophoric gland complex, which packages the sperm into a packet known as a spermatophore. The hectocotylus is a modified arm on the males of many types of cephalopods that is used to transfer spermatophores to the female. The modifications of the arm take many forms, some quite bizarre. The males of some species also exhibit modifications of other arms in addition to the hec-tocotylus. Females of some species also develop such modified structures as arm-tip photophores when mature. Other forms of sexual dimorphism vary among species within families. These forms include such characters as greatly elongated arm pairs or tails; enlarged suckers; and special photophores. Although researchers have speculated about the functions of these features, little is known about them as of 2003.
Spermatophores are implanted in specific locations on the females by the male hectocotylus in species possessing one, or by an elongated penis (the terminal organ of the sperm duct) in nonhectocotylized species. The time required for the process ranges from seconds in some squids to hours in some octopods. The spermatophores may be implanted in the female’s oviducal gland; inside the mantle cavity; around the mantle opening on the neck; in a pocket under the eye; or around the mouth. The mode of reproduction and egg-laying for many cephalopods is unknown, especially oceanic and deep-sea species.
All cephalopod eggs have substantial amounts of yolk. Embryonic development is unlike that of any other mollusk. Nau-tilid eggs are as much as 1.14 in (2.9 cm) in length. Neocoleoid eggs vary in size from about 1.6 in (4.2 cm) in some Granele-done and Megaledone species (among the largest of any invertebrate) to 0.03 in (0.8 mm) long in Argonauta. The eggs have one or more layers of protective coatings and generally are laid as egg masses. Egg masses may be benthic (laid on the ocean bottom) or pelagic, varying among major taxonomic groups. The time span of embryonic development also varies widely from a few days to many months, depending on the species and temperature conditions. Hatching may occur synchronously from a single clutch or extend over a period of 2-3 weeks.
Except for nautilids, which reproduce repeatedly over a period of years, extant cephalopods apparently mate only once. Their reproductive period, however, may comprise the brief terminus of their life span, may extend for a considerable period, or may be intermediate between these extremes. Different species, even within a single family, are found at different points along this continuum but generally cluster at the two extremes. Similarly, the number of eggs produced by a female range from a few dozen to hundreds of thousands. Hatchlings from benthic eggs may either be benthic or temporarily planktonic, eventually settling back to the adult benthic habitat. Pelagic hatchlings are planktonic.
The development of cephalopod embryos is direct, which means that the embryos do not have true metamorphic stages. The hatchlings of species with large eggs look like miniatures of the adult, whereas hatchlings of species with small eggs undergo changes in body proportion during development. The young of some species differ conspicuously in the proportion of their body parts and development of specialized structures (e.g., photophores; modification of suckers into hooks) from the adults. Thus, researchers have coined the term “paralarva” for early stages of cephalopods that differ morphologically and ecologically from later stages.
Most incirrate octopods and, as has recently been discovered, some squids care for their egg masses until the eggs hatch. Pelagic species that exhibit such behavior swim while holding the egg mass in their arms. Benthic octopods use such structures as the interiors of mollusk shells, rock crevices, or discarded bottles as dens. They manipulate the eggs to keep them free of detritus and blow water across them, presumably to aerate them.
Reproduction, at least among coastal species, is seasonal, although there may be either one or two peak periods of seasonal reproduction. Because of their short life spans, neo-coleoid cephalopods tend to exhibit strong seasonality in the occurrence of their different life-history stages.
Conservation status
Distribution and conservation status vary among cephalo-pod species. Some coastal species are quite common whereas others, especially among the oceanic fauna, are rarely encountered. Small-scale endemism (confinement to a specific locality) is uncommon, although endemism is established among cephalopods within ocean basins.
No cephalopods appear on the IUCN Red List. In addition, no cephalopods appear on any United States regional listings as of 2003, although there has been discussion of proposing nautilids for listing under CITES. Most large declines in cephalopod abundance are probably cyclic in nature, although overfishing has been implicated in the declines of commercially important species. The decline in abundance of nautilids probably results from human harvesting of them for both shells and meat.
The abundance of cephalopods varies (depending on group, habitat, and season) from isolated territorial individuals (primarily benthic octopods and sepioids) through small schools with a few dozen individuals to huge schools with millions of individuals.
Significance to humans
Cephalopods have figured in the art and literature of many human cultures. Stories of large or intelligent cephalopods are well known from many ocean-oriented cultures, including Japanese, Polynesian, and Western European. Octopods are depicted on classical Greco-Roman pottery, frescoes, and similar artifacts. Medieval Germanic legends about “Kraken” or sea monsters probably refer to giant squids. Even at the present time, large squids and octopods continue to be subjects of much public interest. For example, the giant squid was described in a special issue of Time magazine on ocean exploration as “the last bona fide sea monster.” This interest has been sustained by a series of popular novels and movies.
Many species of squid, cuttlefish, and octopod are very good food when prepared properly. Many people around the world enjoy dishes made from certain species of cephalopods. Fishermen also often equate cephalopods with “good bait” because other marine species like to eat them as well. These culinary considerations, both human and otherwise, reflect the importance of cephalopods in commercial fisheries and marine food webs. Although worldwide fisheries for squids, cuttlefishes, and octopods are dwarfed in comparison with those for shrimps, bivalves, and many fishes, catching and marketing cephalopods can dominate such local economies as that of the Falkland Islands. The total cephalopod catch officially reported for the year 2000 was about 4.0 million tons (3.6 million metric tons); this figure represents about 4.2% of the world total marine catch for the same year. Some statistics indicate that as humans reduce the populations of competing and predatory fishes and mammals, regional populations of cephalopods are increasing.
Octopods occasionally are considered to be pests in trap fisheries for mollusks (e.g., whelk) and crustaceans (e.g., lobsters) because the octopods enter the traps and eat the catch.
The rediscovery of squid giant axons by J. Z. Young in the 1930s allowed experiments that demonstrated much of what is known about the basic functioning of nerves. The giant ax-ons of squid nerves have been widely used as one of the primary bases of neurobiology. Cephalopods are also being used increasingly as models in other biomedical fields, including sensory biology, information processing, and biochemistry. They are even being used as a source of information regarding the detoxification of nerve gas. The results of an electronic search of scientific literature for the word “squid” may be dominated by biomedical studies based on inshore squid as a convenient and interesting model.
Cephalopod bites, especially by octopods, can be painful at the least. Some are poisonous because of the injection of salivary secretions, and may even be lethal on rare occasions. Poisonous bites from the small blue-ringed octopus, Hapalochlaena spp., which secretes a substance known as cephalotoxin, have resulted in documented human deaths. Furthermore, schools of the large Humboldt squid, Dosidicus gigas, are reported to attack scuba divers and fishermen who have fallen into the water.
Many marine animals of particular interest to people, like whales, specialize in eating cephalopods. Among the animals with large brains that people consider intelligent, the cephalopods are unique because they are not vertebrates. The cephalopod brains and their remarkably familiar eyes have developed from an early precursor completely unlike the forerunners of dogs, dolphins, parrots, lizards, and fishes. This evolutionary history makes cephalopods the closest thing to an alien intelligence that humans have ever encountered.
Because cephalopods are important to biomedical researchers and fisheries, cephalopod biology is comparatively well-known from a few inshore species that can be caught close to such major marine biological laboratories as those in the northeastern United States; Plymouth, England; Naples, Italy; and Hakodate, Japan.

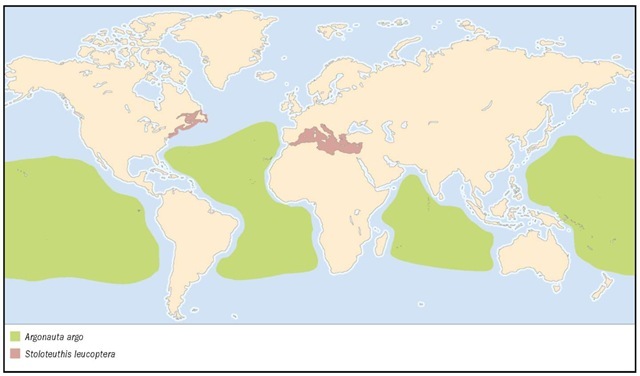
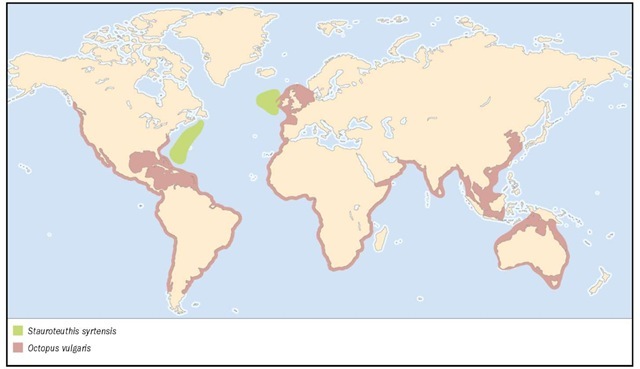
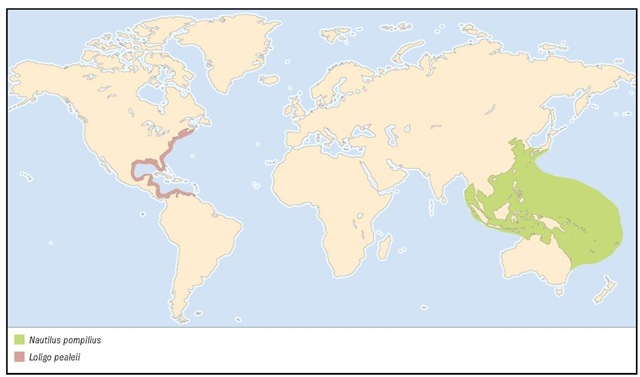
1. Mastigoteuthis magna; 2. Common cuttlefish (Sepia officinalis); 3. Longfin inshore squid (Loligo pealeii); 4. Ram’s horn squid (Spirula spir-ula); 5. Vampire squid (Vampyroteuthis infernalis); 6. Butterfly bobtail squid (Stoloteuthis leucoptera); 7. Pearly nautilus (Nautilus pompilius); 8. Stauroteuthis syrtensis; 9. Greater argonaut (Argonauta argo); 10. Common octopus (Octopus cf. vulgaris).
Species accounts
Longfin inshore squid
Loligo pealeii
ORDER
Myopsida
FAMILY
Loliginidae
TAXONOMY
Loligo pealeii LeSueur, 1821.
OTHER COMMON NAMES
English: Longfinned squid; French: Calmar.
PHYSICAL CHARACTERISTICS
A transparent skin (corneal membrane) covers eye lens. Funnel-locking apparatus is a simple, straight groove and ridge. Fins attach to lateral regions of mantle. Arms have suckers in two series. Tentacular club with suckers in four series. Hooks are never present. Buccal connectives attach to ventral margins of ventral arms. Seven buccal lappets possess small suckers. Mantle is long, moderately slender, cylindrical, with a bluntly pointed posterior end; the fins are rhomboid with nearly straight sides. The gladius is long, rather wide, and feather-shaped; the ratio of greatest width of the vane of the gladius to greatest width of the rhachis (the central spine of the gladius) is 2.7-3.7 in females, 2.4-2.9 in males. The edge of the vane is curved (sometimes straight in males), thin, and rarely ribbed. Eyes are not unusually large; the diameter of the externally visible eyeball is 8-18% ML, and the diameter of dissected lens is 2-6% ML. The left ventral arm of mature males is hectocotyl-ized by modification of the outermost third to fourth of arm, but the modification does not extend to arm tip. Fewer than 12 of the suckers in dorsal row usually smaller than half the size of their counterparts in the ventral row; the bases or pedicels of some of the modified suckers are rounded and narrowly triangular.
DISTRIBUTION
Western Atlantic continental shelf and upper slope waters from Nova Scotia to Venezuela, including the Gulf of Mexico and the Caribbean Sea. Not found around islands except as rare strays at islands close to the continental shelf or slope.
HABITAT
Restricted in summer to surface and shallow water, but lives at a depth of 92-1,200 ft (28-366 m) in winter; peak concentrations occur at 328-633 ft (100-193 m); adults are found on the bottom during day but leave the bottom at night, dispersing into the water column. They may appear at the water surface in summer or in warm water.
BEHAVIOR
Longfin inshore squid have a summer, inshore-northerly spawning migration to shallow coastal and shelf waters, fol-
lowed by an offshore-southerly retreat in fall and winter to the waters of the continental slope.
FEEDING ECOLOGY AND DIET
Food includes crustaceans, fishes, and squids.
REPRODUCTIVE BIOLOGY
Eggs are laid in gelatinous finger-like strands, many of which are attached together in large masses (“sea mops”) to a solid substrate at depths from a few feet to 820 ft (250 m). The par-alarvae are planktonic.
CONSERVATION STATUS
Not listed by the IUCN. Occasionally of concern because of potential overfishing.
SIGNIFICANCE TO HUMANS
A very important species for commercial fisheries. Has been used extensively in behavioral and neurological experiments.
Pearly nautilus
Nautilus pompilius
ORDER
Nautilida
FAMILY
Nautilidae
TAXONOMY
Nautilus pompilius Linnaeus, 1758.
OTHER COMMON NAMES
English: Chambered nautilus, common nautilus, emperor nautilus, flame nautilus; French: Nautile chambre, nautile naturel; German: Perlboot, Schiffsboot; Italian: Nautilo.
PHYSICAL CHARACTERISTICS
External chambered shell, umbilicus filled in with a concretion; eyes form images via a pinhole opening; funnel formed by overlapping flaps; flame-striped color pattern extending across nearly entire shell.
DISTRIBUTION
Indo-West Pacific.
HABITAT
Continental shelf and slope from near the surface to a depth of about 2,460 ft (750 m). Associated with hard ocean bottoms, particularly coral reefs. Undergoes diel vertical migrations.
BEHAVIOR
Activity primarily nocturnal.
FEEDING ECOLOGY AND DIET
Feeds on sedentary benthic prey and carrion (dead or decaying animal flesh).
REPRODUCTIVE BIOLOGY
Reproduces repeatedly over a period of years; attaches large eggs with irregularly shaped coverings to hard substrates.
CONSERVATION STATUS
Not listed by the IUCN. Of concern because of pressure from locally heavy fishing.
SIGNIFICANCE TO HUMANS
Fished for meat and shells, which are valuable to collectors.
Greater argonaut
Argonauta argo
ORDER
Octopoda
FAMILY
Argonautidae
TAXONOMY
Argonauta argo Linnaeus, 1758.
OTHER COMMON NAMES
English: Paper nautilus; French: Argonaute, argonaute papier; German: Papierboot; Spanish: Argonauta comun.
PHYSICAL CHARACTERISTICS
Has a locking apparatus for the funnel and mantle that consists of a knob and pit. Lacks water pores. Mature females produce an external shell-like egg case. Females have a flag-like expansion of the web of the dorsal arms that contains shell-secreting glands. The male hectocoty-lus develops in a sac beneath the eye; lacks a lateral papillate fringe.
DISTRIBUTION
Open ocean in tropical and subtropical regions.
HABITAT
Surface waters; rarely encountered near shore.
BEHAVIOR
Has been found attached to jellyfish that it uses as a source of food and protection. Males have been reported living within salps (free-swimming tunicates).
FEEDING ECOLOGY AND DIET
Feeds on crustaceans and jellyfish.
REPRODUCTIVE BIOLOGY
The male argonaut is a dwarf, about 10% of the length of the female. The entire third right arm is hectocotylized and carried in a special sac. At mating, the hectocotylus, which carries one large spermatophore, breaks out of its sac and free from the male body. It then invades or is deposited in the female’s mantle cavity, where it remains viable and active for some time. Females brood many thousands of very small eggs deposited in an external shell-like egg case.
CONSERVATION STATUS
SIGNIFICANCE TO HUMANS
The egg cases are valuable to shell collectors.
Common octopus
Octopus cf vulgaris
ORDER
Octopoda
FAMILY
Octopodidae
TAXONOMY
Octopus vulgaris Cuvier, 1797. Although Octopus vulgaris has been reported to be widely distributed around the world, researchers interested in cephalopod systematics have long known that these reports represent a species complex. Unfortunately, the relationships among the various populations within this complex have not yet been resolved.
OTHER COMMON NAMES
English: Devilfish, scuttle; French: Pieuvre, poulpe commun; German: Gewohnlicher Krake, Oktopus, Seepolyp; Portuguese: Polvo; Spanish: Pulpo comun, pulpo de roca.
PHYSICAL CHARACTERISTICS
Has two series of suckers. Structures (when present) on dorsal surfaces of mantle, head, arms not star-shaped cartilaginous tubercles. Funnel organ is W-shaped. Ink sac present. No ocelli between eye and bases of lateral arms. Has 9-11 gill lamellae on each outer demibranch. Mantle opening is wide. Dorsal arms are shortest; lateral arms longer than ventral arms; ven-trolateral arms only slightly longer than dorsolateral arms.
Character states for distinguishing among species in this group await publication of redescriptions.
DISTRIBUTION
This species group is found worldwide in temperate and tropical waters; the true O. vulgaris is probably restricted to European and northern African waters.
HABITAT
A benthic species occurring from the coastline to the outer edge of the continental shelf in depths from 0-656 ft (0-200 m), where it is found in a variety of habitats, including rocks, coral reefs, and seagrass beds. The paralarvae are planktonic.
BEHAVIOR
The complex behavior of these octopods has been described in great detail. Although researchers disagree about the level of intelligence of these mollusks, or even how to measure it, they have referred to these animals as the closest thing to alien intelligence encountered by humans.
FEEDING ECOLOGY AND DIET
Feeds primarily on crustaceans (crabs and lobsters) and mol-lusks (clams and snails). Females may produce between 120,000 and 400,000 eggs little longer than 0.07 in (2 mm), which they deposit in strings in crevices or holes, usually in shallow waters. Spawning may take as long as a month. During the brooding period (25-65 days, depending on water temperature), the females almost stop feeding; many die after the hatching of the paralarvae.
REPRODUCTIVE BIOLOGY
Two peak periods of spawning, in the spring and autumn of each year.
CONSERVATION STATUS
Not listed by the IUCN. Occasionally of concern because of potential overfishing.
SIGNIFICANCE TO HUMANS
A very important species for commercial fisheries. Has also been used extensively in behavioral experiments. ♦
No common name
Stauroteuthis syrtensis
ORDER
Octopoda
FAMILY
Stauroteuthidae
TAXONOMY
Stauroteuthis syrtensis Verrill, 1879.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
Fins present. Cirri on arms. One series of suckers along the entire length of the arm. No filamentous appendages in pouches between the bases of the dorsal and dorsolateral arms. Light organ is present at base of each sucker. Contractile intermediate membrane or secondary web is present between each arm and the primary web. Shell is cartilaginous and has a simple U-shape. Gills are sepioid.
DISTRIBUTION
North Atlantic Ocean.
HABITAT
Found near bottom at depths of 1,640-13,125 ft (5004,000 m) with maximum abundance between 4,920-8,200 ft (1,500-2,500 m).
BEHAVIOR
Has been observed drifting passively with the arms and web held in a bell-shaped posture. Swims by flapping its fins rather than jetting.
FEEDING ECOLOGY AND DIET
Feeds on small crustaceans that may be entangled in mucus produced by glands around the mouth.
REPRODUCTIVE BIOLOGY
Eggs of various sizes in ovary indicate slow and prolonged spawning rather than spawning of synchronous batches.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
No common name
Mastigoteuthis magna
ORDER
Oegopsida
FAMILY
Mastigoteuthidae
TAXONOMY
Mastigoteuthis magna Joubin, 1913.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
Ventral arms are elongated. Tentacles are vermiform (wormlike); clubs not expanded or only slightly expanded; moderate in length to very elongate with minute suckers in many series. Funnel locking apparatus is oval with knobs affecting the shape. Fins are very large and positioned mostly behind the muscular part of the mantle. This cephalopod is weakly muscled and reddish in color; much of the red pigment is not located in chromatophore organs but is dispersed among other cells in the organism’s skin.
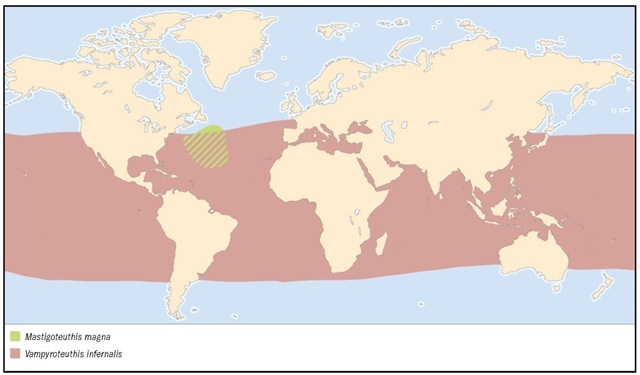
DISTRIBUTION
Tropical and northern subtropical Atlantic; also reported from the Indian Ocean and Tasman Sea.
HABITAT
Bathypelagic and bathyal depths (between 600-13,000 ft or 200-4,000 m).
BEHAVIOR
Has been observed from submersibles drifting in a vertical posture just above the ocean floor. The organism’s ventral arms were spread and held the tentacles, which dangled downward very close to the bottom.
FEEDING ECOLOGY AND DIET
Feeds on small planktonic crustaceans.
REPRODUCTIVE BIOLOGY
Not studied.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
Important part of food webs on the continental slope that support commercial finfish fisheries.
Common cuttlefish
Sepia officinalis
ORDER
Sepioidea
FAMILY
Sepiidae
TAXONOMY
Sepia officinalis Linnaeus, 1758.
OTHER COMMON NAMES
French: Casseron, seiche commune; German: Gemeine Sepie, Gemeine Tintenfisch; Italian: Seppia comune; Spanish: Casta-fuela, sepia comun.
PHYSICAL CHARACTERISTICS
Internal shell is a cuttlebone. Cuttlebone length approximately equals mantle length. Mantle is elongated and oval in shape. Fins extend almost the full length of mantle. Tentacles can be completely withdrawn into pockets. Tentacular club is short, narrow, and crescent-shaped. Eye lens is covered by a cornea.
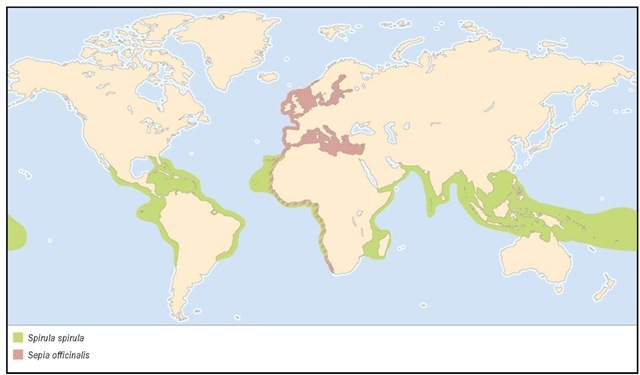
DISTRIBUTION
Eastern Atlantic: from the Baltic and North Seas to South Africa; Mediterranean Sea.
HABITAT
A demersal (swimming near bottom) species occurring predominantly on sandy to muddy bottoms from the coastline to about 656 ft (200 m) depth, but most abundant at less than 328 ft (100 m).
BEHAVIOR
As with O. vulgaris, the behavior of cuttlefish is very complex and has been studied in great detail.
FEEDING ECOLOGY AND DIET
Feeds on small mollusks, crabs, shrimps, other cuttlefishes, and juvenile demersal fishes. Cannibalism is common and has been interpreted as a strategy to overcome temporary shortages of adequately sized prey.
REPRODUCTIVE BIOLOGY
Males may carry as many as 1,400 spermatophores, and females produce between 150-4,000 eggs, depending on their size. The eggs measure from 0.31-0.39 in (8-10 mm) in diameter and
are attached in grapelike clusters to seaweeds, debris, shells and other substrates. They hatch after 30-90 days depending on temperature— 70.7-59°F (21.5-15°C, respectively). Hatchlings are large (0.27-0.31 in or 7-8 mm) dorsal mantle length and look like miniature versions of adults.
CONSERVATION STATUS
Not listed by the IUCN. Occasionally of concern because of potential overfishing.
SIGNIFICANCE TO HUMANS
A very important species for commercial fisheries. Has also been used extensively in behavioral experiments. At one time the ink produced by the cuttlefish was used as a dye.
Butterfly bobtail squid
Stoloteuthis leucoptera
ORDER
Sepioidea
FAMILY
Sepiolidae
TAXONOMY
Stoloteuthis leucoptera Verrill, 1878.
OTHER COMMON NAMES
None known.
PHYSICAL CHARACTERISTICS
Mantle is short, and rounded toward the rear. Fins are broadly separated toward the rear, with free anterior and posterior lobes. The gladius is rudimentary. There is a median mantle septum with strong adductor muscles. The eye is covered by a cornea; there is a ventral eyelid present. The arms lack protective membranes. The dorsal mantle is fused to the head; the area of fusion is narrower than the width of the head. Posterior edges of fins do not extend beyond posterior mantle. Ventral shield of mantle does not extend beyond middle of eyes. Males have enlarged suckers on their dorsolateral arms.
DISTRIBUTION
North Atlantic off the eastern United States; Mediterranean Sea.
HABITAT
Continental shelf and slope at depths of 575-1,115 ft (175— 340 m).
BEHAVIOR
Has been observed hovering in midwater far above the bottom, rapidly beating its fins.
FEEDING ECOLOGY AND DIET
Not studied; probably feeds on small planktonic crustaceans.
REPRODUCTIVE BIOLOGY
Nothing is known.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
None known.
Ram’s horn squid
Spirula spirula
ORDER
Sepioidea
FAMILY
Spirulidae
TAXONOMY
Spirula spirula Linnaeus, 1758. Some experts assign this species to a separate order, Spirulida.
OTHER COMMON NAMES
French: Spirule; German: Posthorn.
PHYSICAL CHARACTERISTICS
Partially internal shell is curved ventrally in an open coil; each coil is round in cross section and has transverse septa (dividers) with a siphuncle. There are four series of suckers on the arms. Both ventral arms are hectocotylized in males. There are tentacular clubs with suckers in 16 series; not divided into manus and dactylus. Eyes lack a cornea. Fins are separate, terminal, and lie in a plane nearly transverse to body axis. Large pho-tophore at rear end of body.
DISTRIBUTION
Mesopelagic waters of open tropical oceans.
HABITAT
Found at depths between 1,800—3,280 ft (550—1,000 m), mostly over the slopes of continents or islands where the ocean bottom lies between 3,280—6,560 ft (1,000—2,000 m).
BEHAVIOR
Captive Spirula hang head-downward in the water. This species is able to withdraw its head and arms completely within the mantle; the mantle opening can then be closed by folding over the large dorsal and ventrolateral extensions of the mantle margin. The photophore at the posterior end of the body is known to glow for hours at a time. When the animal is swimming slowly downward, head first, its terminal fins are pointed upward (i.e. posteriorly) and move with a rapid “waving or fluttering motion” that propels it downward (Bruun, 1943).
FEEDING ECOLOGY AND DIET
Nothing is known.
REPRODUCTIVE BIOLOGY
It has been suggested that Spirula lays its eggs on the ocean floor; the capture of very small animals in deep water supports this hypothesis.
CONSERVATION STATUS
Not listed by the IUCN.
SIGNIFICANCE TO HUMANS
The shells, which commonly wash ashore in some areas, are prized by collectors.
Vampire squid
Vampyroteuthis infernalis
ORDER
Vampyromorpha
FAMILY
Vampyroteuthidae
TAXONOMY
Vampyroteuthis infernalis Chun, 1903.
OTHER COMMON NAMES
French: Vampire des enfers; German: Vampir-Tintenfisch.
PHYSICAL CHARACTERISTICS
Has retractile filaments extending from pockets between the dorsal and dorsolateral arms. Fins present. Large circular, lidded photophores present behind each adult fin (“fin-base” organs); numerous small photophores distributed over the lower surfaces of mantle, funnel, head and the aboral surface of the arms and web (“skin-nodule” organs). A gladius, or internal remnant of an original external shell, is present with a broad median field and conus (cup-shaped tip). Cirri are present over the entire length of the arm; suckers lack a cuticular lining and are present only on the outer half of arms.
DISTRIBUTION
Found throughout tropical and temperate oceans.
HABITAT
Meso to bathypelagic depths, generally 1,965—4,920 ft (600—1,500 m).
BEHAVIOR
Can swim surprisingly fast for a gelatinous animal. Arms are sometimes spread forward to form, along with the web, an umbrella-like or bell-shaped posture. Filaments appear to be tactile sense organs. Uses combinations of photophores for complex luminescent displays.
FEEDING ECOLOGY AND DIET
Poorly understood; diet includes gelatinous megaplankton.
REPRODUCTIVE BIOLOGY
No hectocotylus. Early development passes through stages with (1) a single pair of larval fins; (2) two pairs of fins, larval plus adult; and (3) a single pair of adult fins.
CONSERVATION STATUS
Not listed by the IUCN. Only a single extant species has been described in this order.
SIGNIFICANCE TO HUMANS
Frequently featured in natural-history television programs.