Diagnostic Strategy
Algorithms A number of prospectively validated algorithms have been published that emphasize the use of different initial noninvasive tests in conjunction with ventilation-perfu-sion lung scanning. The noninvasive tests, as used in these algorithms, are as follows: structured clinical assessment and serial venous ultrasounds50; empirical clinical assessment, sensitive D-dimer assay, and venous ultrasound at presentation only42; and clinical assessment, moderately sensitive D-dimer assay, and serial venous ultrasounds57 [see Figure 4]. Helical CT can be used rather than ventilation-perfusion scanning; Musset and colleagues have described and validated a diagnostic algorithm for pulmonary embolism that uses routine helical CT, ultra-sonography of the proximal veins, and clinical assessment.54
Evaluation of patients with nondiagnostic, noninvasive tests Serial venous ultrasonography of the proximal veins (i.e., 1 and 2 weeks after the initial evaluation) is suitable for most patients in whom noninvasive tests are not diagnostic,50,56 although pulmonary angiography is generally preferred for certain subgroups (see below). An alternative to pulmonary an-giography is to perform bilateral venography before serial venous ultrasonography.
Pulmonary angiography is the preferred option in patients with a segmental intraluminal filling defect on helical CT. However, when clinical suspicion is high, this CT finding alone is likely to have a positive predictive value of 85% and could be considered diagnostic for pulmonary embolism. A subsegmen-tal intraluminal filling defect on helical CT and high clinical probability of pulmonary embolism is also an indication for an-giography, as is a high-probability ventilation-perfusion scan result and low clinical suspicion. Alternatively, helical CT scanning can be followed with ventilation-perfusion scanning, or vice versa, when the initial scan shows these findings; in such cases, the second test may be diagnostic for pulmonary embolism. If the second test is also nondiagnostic for pulmonary embolism, serial ultrasonography may be reconsidered.
Pulmonary angiography is also preferred when clinical manifestations are severe, posttest probability of pulmonary embolism is moderate, and pulmonary embolism needs to be excluded from the differential diagnosis. Finally, pulmonary angiography is indicated when serial testing is not feasible (e.g., because of impending surgery or geographic inaccessibility).
Prophylaxis and Treatment
Pharmacology of antithrombotic agents
Anticoagulants
A less intense anticoagulant effect is required for the prevention of venous thrombosis than is required for its treatment. The anticoagulants in clinical use are heparin, LMWH, and fon-daparinux, which are administered subcutaneously or intravenously; and coumarin compounds, which are given orally. Thrombolytic agents are streptokinase, urokinase, and recom-binant tissue plasminogen activator (rt-PA).
Heparin and LMWH Heparin is a highly sulfated gly-cosaminoglycan that produces its anticoagulant effect by binding to antithrombin, markedly accelerating the ability of the naturally occurring anticoagulant to inactivate thrombin, activated factor X (factor Xa), and activated factor IX (factor IXa).59 At therapeutic concentrations, heparin has a half-life of about 60 minutes. Its clearance is dose dependent. Heparin has decreased bioavailability when administered subcutaneously in low doses but has approximately 90% bioavailability when administered in high therapeutic doses.
Heparin binds to a number of plasma proteins, a phenomenon that reduces the anticoagulant effect of heparin by limiting its accessibility to antithrombin. The concentration of heparin-binding proteins increases during illness, contributing to the variability in anticoagulant response in patients with throm-boembolism.59 Because of this variability, response to heparin should be monitored with the activated partial thromboplastin time (aPTT). The dose should be adjusted as necessary to achieve a therapeutic range, which for many aPTT reagents corresponds to an aPTT ratio of 1.5 to 2.5.
LMWHs are effective in the prevention and treatment of venous thrombosis. They are derived from standard commercial-grade heparin by chemical depolymerization to yield fragments approximately one third the size of heparin.60 Depolymerization of heparin results in a change in its anticoagulant profile, bioavailability, and pharmacokinetics and in a lower incidence of heparin-induced thrombocytopenia and of osteopenia.59,60
The plasma recoveries and pharmacokinetics of LMWHs differ from those of heparin because LMWHs bind much less avidly to heparin-binding proteins than does heparin. This property of LMWHs contributes to their superior bioavailability at low doses and their more predictable anticoagulant response. LMWHs also exhibit less binding to macrophages and endothelial cells than does heparin, a property that accounts for their longer plasma half-life, which is approximately 3 hours, and their dose-independent clearance. These potential advantages over heparin permit once-daily administration of LMWHs without laboratory monitoring. The advantages of LMWHs have been exploited to successfully treat patients with DVT out of hospital61-63 and to treat patients with acute pulmonary embolism in hospital with once-or twice-daily subcutaneous dosing regimens.47,63 The published research on LMWHs, which includes over 3,000 patients treated with either once-daily or twice-daily subcutaneous doses, has established this class of anticoagulants as safe, effective, and convenient for treating venous thrombosis and pulmonary embolism.28
Fondaparinux Fondaparinux is a new parenteral synthetic anticoagulant composed of the five saccharide units that make up the active site of heparin that binds antithrombin.
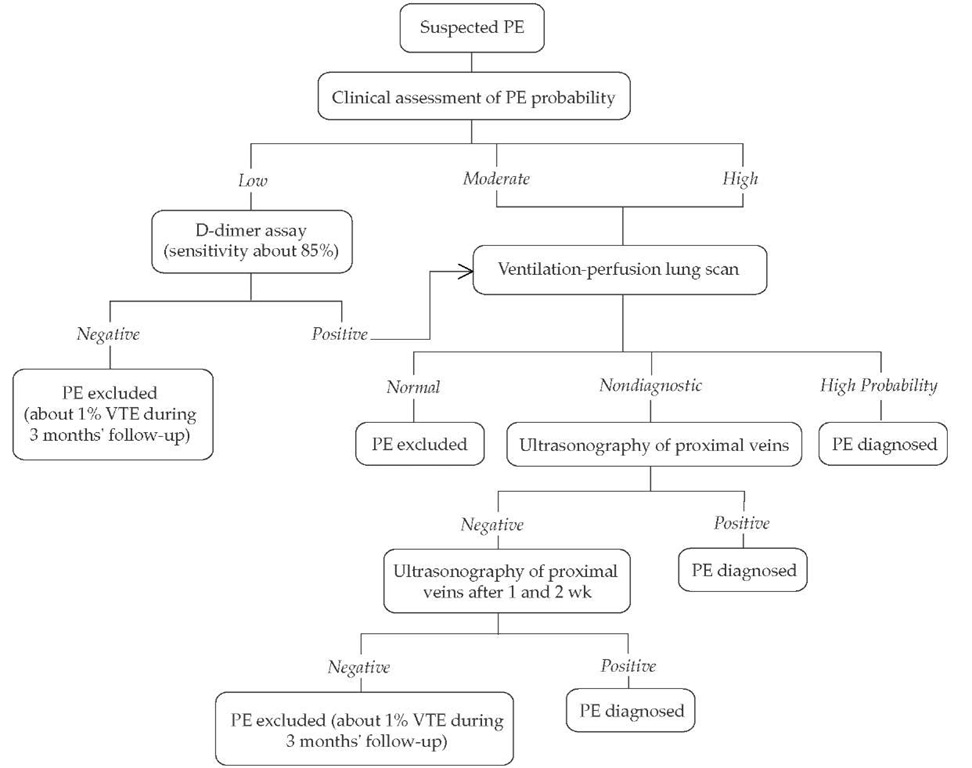
Figure 4 Diagnostic approach to pulmonary embolism. Use of a very sensitive D-dimer assay can obviate clinical assessment: a negative result excludes pulmonary embolism regardless of the clinical assessment results; a positive test can be followed by a ventilation-perfusion scan. Similarly, a ventilation-perfusion scan can substitute for clinical assessment.
If the clinical assessment indicates a low probability of pulmonary embolism and, in particular, if a D-dimer test is not done, pulmonary angiography or helical CT may be considered. If helical CT is used instead of ventilation-perfusion lung scanning, intraluminal filling defects in segmental or larger pulmonary arteries are generally diagnostic for pulmonary embolism, but all other findings are nondiagnostic, including normal results and intraluminal filling defects confined to the subsegmental pulmonary arteries. With nondiagnostic results, management is the same as with a nondiagnostic lung scan.
If ultrasonography is negative but overall assessment suggests a high probability of pulmonary embolism, symptoms are severe, or cardiopulmonary reserve is poor, then additional tests (e.g., helical CT or bilateral venography) may be considered. Venography should be considered if there is an increased risk of a false-positive ultrasound result (e.g., previous venous thromboembolism, equivocal ultrasound findings, preceding findings suggesting a low probability of pulmonary embolism). It is reasonable to not repeat ultrasound testing, or to do only one more ultrasound after 1 week, if preceding findings suggest a low probability of pulmonary embolism. (PE—pulmonary embolism; VTE—venous thromboembolism)
Fondaparinux is rapidly absorbed and is 100% bioavailable when administered subcuta-neously. It is not metabolized, is renally excreted, and has a dose-independent elimination half-life of 15 hours, which makes it suitable for once-daily administration. Fondaparinux is being evaluated for many indications, including prevention and treatment of DVT and pulmonary embolism.
Oral anticoagulants Oral anticoagulants are coumarin compounds, the most common being warfarin, that produce their anticoagulant effect through the production of hemostati-cally defective, vitamin K-dependent coagulant proteins (prothrombin, factor VII, factor IX, and factor X).65
The dose of warfarin must be monitored closely because the anticoagulant response varies widely among individuals. Laboratory monitoring is performed by measuring the prothrombin time (PT), a test responsive to depression of three of the four vitamin K-dependent clotting factors (prothrombin and factors VII and X). Commercial PT reagents vary markedly in their responsiveness to warfarin-induced reduction in clotting factors. This problem of variability in the responsiveness of PT reagents has been overcome by the introduction of the international normalized ratio (INR).65
The starting dose of warfarin has been 10 mg, with an average maintenance dose of about 5 mg. However, the dose required varies widely among individuals. Elderly patients, for example, have been shown, on average, to require lower doses. Evidence indicates that it might be safer to use a starting dose of 5 mg of warfarin because, compared with 10 mg, the 5 mg starting dose does not result in a delay in achieving a therapeutic INR and is associated with a lower incidence of supratherapeutic INR values during the first 5 days of treat-ment.66 Warfarin therapy is difficult to manage in some patients because of unexpected fluctuations in dose response, which may reflect changes in diet, inaccuracy in PT testing, undisclosed drug use, poor compliance, or surreptitious self-medication. Certain over-the-counter and prescription drugs can augment or inhibit the anticoagulant effect of coumarin compounds or prolong hemostasis by interfering with platelet function [see Table 5].
Patients receiving coumarin compounds are also sensitive to fluctuating levels of dietary vitamin K, which is obtained predominantly from leafy green vegetables. The effect of coumarins can be potentiated in sick patients with poor vitamin K intake, particularly if they are treated with antibiotics and intravenous feeding without vitamin K supplementation, and in states of fat malabsorption.
Direct thrombin inhibitors Direct thrombin inhibitors include hirudin, bivalirudin, argatroban, melagatran, and ximela-gatran.67 Whereas the first four of these compounds must be administered parenterally, ximelagatran is absorbed from the gastrointestinal tract and exhibits no known food or drug interactions. Once absorbed, ximelagatran is converted to mela-gatran, a partial mimetic of fibrinopeptide A, which blocks the active site of thrombin. Ximelagatran is primarily eliminated by the kidneys and has a half-life of about 3 hours, mandating twice-daily administration. It is being evaluated for many indications, including primary prevention and acute and long-term treatment of venous thromboembolism.
Table 5 Drug and Food Interactions with Warfarin by Level of Supporting Evidence* and Direction of Interaction92
|
|
Antibiotics |
Cardiac |
Anti-inflammatory |
Central Nervous System |
Gastrointestinal |
Miscellaneous |
|
Potentiation Level 1 |
Trimethoprim-sulfamethoxazole, erythromycin, isoniazid, fluconazole, metronidazole, miconazole, clarithromycin, amoxicillin |
Amiodarone, clofibrate, propafenone, propranolol, sulfinpyrazone+ |
Phenylbutazone,+ piroxicam High-dose intravenous methylprednisolone Acetaminophen |
Alcohol (with liver disease) |
Cimetidine,* omeprazole |
|
|
Level 2 |
Ciprofloxacin, itraconazole, tetracycline, norfloxacin |
Aspirin, quinidine, simvastatin |
Aspirin, dextro-propoxyphene |
Chloral hydrate, disulfiram, phenytoin |
— |
Anabolic steroids, influenza vaccine, tamoxifen |
|
Level 3 |
Nalidixic acid, ofloxacin |
Disopyramide, lovastatin, metolazone |
Topical salicylates, sulindac, tolmetin |
— |
— |
Fluorouracil, ifosfamide |
|
Level 4 |
Cefamandole, cefazolin, sulfisoxazole, doxycycline |
Gemfibrozil, heparin |
Indomethacin |
|||
|
Inhibition Level 1 |
Griseofulvin,* nafcillin, rifampin |
Cholestyramine |
Barbiturates, carbamazepine, chlordiazepoxide |
Sucralfate |
Foods with a high vitamin K content, enteral nutritional support, large amounts of avocado Ticlopidine |
|
|
Level 2 |
Dicloxacillin |
— |
— |
— |
— |
— |
|
Level 3 |
Azathioprine |
Trazodone |
Azathioprine, cyclo-sporine, etretinate, large amounts of broccoli |
|||
|
No effect Level 1 |
Enoxacin |
Atenolol, bumetanide, felodipine, metoprolol, moricizine |
Diflunisal, ketorolac, naproxen |
Alcohol, fluoxetine, nitrazepam |
Antacids, famotidine, nizatidine, psyllium, ranitidine* |
|
|
Level 2 |
Ketoconazole |
— |
Ibuprofen, ketoprofen |
— |
— |
— |
|
Level 4 |
Vancomycin |
Diltiazem |
— |
— |
— |
Tobacco |
*Level 1 evidence indicates that the likelihood of an association is very strong; level 2 evidence suggests that a true association is likely; level 3 evidence suggests that a true association is probable; and level 4 evidence suggests that a true association is possible. Supporting level 1 evidence was obtained from both patients and volunteers. tIn a small number of volunteers, an inhibitory drug interaction occurred.
Thrombolytic Agents
Pharmacologic thrombolysis is produced by plasminogen activators—including streptokinase, rt-PA, and urokinase— which convert the proenzyme plasminogen to the fibrinolytic enzyme plasmin.
Streptokinase Streptokinase is a protein produced by |-he-molytic streptococci. In contrast to other plasminogen activators, streptokinase is not an enzyme and does not convert plas-minogen directly to plasmin by proteolytic cleavage. Instead, streptokinase binds noncovalently to plasminogen, converting it to a plasminogen-activator complex that acts on other plas-minogen molecules to generate plasmin. Streptokinase has a plasma half-life of 30 minutes.
Because streptokinase is a bacterial product, it stimulates antibody production and can prompt allergic reactions. Antistrep-tococcal antibodies, present in variable titers in most patients before streptokinase treatment, induce an amnestic response that makes repeated treatment with streptokinase difficult or impossible for a period of months or years after an initial course of treatment. Laboratory monitoring of streptokinase can be limited to thrombin time, which is used as a marker for an effective lytic state. If the thrombin time is not prolonged within the first few hours of commencing treatment, resistance to streptokinase resulting from a high titer of antistreptococcal antibodies should be suspected and the dose of streptokinase should be increased.
Urokinase Synthesized by endothelial and mononuclear cells, urokinase is a direct activator of plasminogen. Like strep-tokinase, urokinase is non-fibrin specific. It has a plasma half-life of 10 minutes.
Complications of Antithrombotic Agents
Bleeding is the main complication of antithrombotic thera-py.69 With all antithrombotic agents, the risk of bleeding is influenced by the dose and by patient-related factors, the most important being recent surgery or trauma. Other patient characteristics that increase the risk of bleeding are older age, recent stroke, generalized hemostatic defect, a history of gastrointestinal hemorrhage, and serious comorbid conditions.
Bleeding is more common and more serious with throm-bolytic drugs than with anticoagulants. The risk of bleeding with thrombolytic therapy is just as great with rt-PA as with streptokinase and urokinase, which, unlike rt-PA, lack fibrin specificity. With heparin, the incidence of bleeding is influenced by dosage and by means of administration, being higher with intermittent intravenous therapy than with continuous intravenous therapy.69 Bleeding rates are similar for heparin and LMWH.28
Bleeding associated with coumarin anticoagulants is influenced by the intensity of anticoagulant therapy. Such bleeding is reduced to about one third if the targeted INR range is lowered from between 3.0 and 4.5 to between 2.0 and 3.0. Both hep-arin-induced bleeding and warfarin-induced bleeding are increased by concomitant use of aspirin, which impairs platelet function and produces gastric erosions. When the INR is less than 3.0, coumarin-associated bleeding frequently has an obvious underlying cause or is from an occult gastrointestinal or renal lesion.
Nonhemorrhagic side effects of thrombolytic therapy are limited mainly to allergic reactions to streptokinase. Nonhem-orrhagic side effects of heparin include the following: (1) urticaria at sites of subcutaneous injection; (2) thrombocytopenia, which occurs in 2% to 4% of patients treated with high-dose heparin and is complicated by arterial or venous thrombosis in about 0.2% of treated patients [see 5:XIV Thrombotic Disorders]; (3) osteoporosis, which occurs with prolonged high-dose hep-arin use; and, rarely, (4) alopecia, adrenal insufficiency, and skin necrosis. The incidence of thrombocytopenia is lower with LMWHs than with heparin. Similarly, there is evidence that the risk of osteopenia is lower with LMWH than with heparin.59
The most important nonhemorrhagic side effect of coumarin anticoagulants is skin necrosis, an uncommon complication usually observed on the third to eighth day of therapy. Skin necrosis is caused by extensive thrombosis of the venules and capillaries within the subcutaneous fat. An association has been reported between coumarin-induced skin necrosis and protein C defi-ciency—and, less commonly, protein S deficiency—but this complication can occur in patients without these deficiencies.