Principles of Antiretroviral Therapy
An expert panel convened by the DHSS and the Henry J. Kaiser Family Foundation has issued antiretroviral therapy recommendations for medical providers in the United States. These guidelines, which are based on data from clinical trials, basic vi-rologic principles, and clinical experience, were last updated March 23, 2004, and will continue to be modified as needed.91 A second set of published guidelines was issued by the International AIDS Society95; these guidelines had more international input than the DHHS guidelines. As described in the DHHS guidelines, the goal of antiretroviral therapy is to obtain maximal and durable suppression of HIV, restore and preserve immune function, improve quality of life, and reduce HIV-related mortality.
Figure 11 This illustration depicts the site of action of four major classes of antiretroviral medications
Initiation of therapy
The DHHS guidelines recommend that antiretroviral therapy be offered to chronically infected patients who have severe HIV-related symptoms or AIDS or who are asymptomatic but meet certain immunologic or virologic thresholds. Asymptomatic patients with CD4+ T cell counts below 200/^l should be offered therapy because of the significant risk of opportunistic infection and death without treatment. Asymptomatic patients with CD4+ T cell counts between 200 and 350/ ^l should generally be offered treatment unless their plasma HIV RNA level is low, in which case their short-term risk of disease progression and death is also low. Asymptomatic patients with CD4+ T cells above 350/^l can generally be followed without treatment unless their HIV RNA level is high (> 55,000 copies/ml by reverse transcriptase polymerase chain reaction [RT-PCR] or branched DNA [bDNA] assay), in which case some providers recommend treatment because of the risk of more rapid disease progression. Patients who are diagnosed during acute infection or within 6 months of infection may have the unique opportunity to modify their disease history with treatment (see above). Theoretically, early treatment may lower the viral set point, preserve HIV-directed CD4+ T cells, relieve symptoms of acute infection, and prevent transmission. These benefits, however, must be weighed against the toxicity of therapy, the possible promotion of drug-resistant HIV strains, and the lack of long-term clinical data demonstrating a clear benefit of early therapy.
REGIMeN SELECtION
Antiretroviral therapy should consist of a potent regimen that includes a combination of three or more medications [see Table 6]. The combination regimens typically consist of (1) two NRTIs plus a protease inhibitor, (2) two NRTIs plus an NNRTI, or (3) two NRTIs plus ritonavir plus a second protease inhibitor. In the last case, ritonavir’s potent inhibition of the cytochrome P-450 elimination pathway is used to boost the blood level of the second protease inhibitor. Preferred regimens for antiretroviral-naive patients have been identified in the DHHS guidelines [see Table 6].
Enfuvirtide is most effective in patients with CD4+ T cell counts above 100 cells/^l; in these cases, enfuvirtide is combined with a standard regimen of drugs that includes one or two agents to which the patient’s virus is sensitive. However, patients who do not meet these criteria may still experience a viro-logic response and some immunologic benefit from enfuvirtide. These benefits must be weighed against the cost, difficulty of administration (twice-daily subcutaneous injections), and adverse effects (e.g., injection-site reactions, hypersensitivity, and possible pneumonia).
Table 4 Antiretroviral Medications
|
Generic Name |
Trade Name |
Dosage |
|
Nucleoside RTIs |
|
|
|
Abacavir (ABC) |
Ziagen |
300 mg p.o., b.i.d. |
|
Didanosine (ddI) |
Videx |
400 mg p.o., q.d. (250 mg q.d. if < 60 kg) |
|
Emtricitabine (FTC) |
Emtriva |
200 mg p.o., q.d. |
|
Lamivudine (3TC) |
Epivir |
150 mg p.o., b.i.d. |
|
Stavudine (d4T) |
Zerit |
40 mg p.o., b.i.d. (30 mg p.o., b.i.d., if < 60 kg) |
|
Zalcitabine (ddC) |
Hivid |
0.75 mg p.o., t.i.d. |
|
Zidovudine (AZT) |
Retrovir |
300 mg p.o., b.i.d.; or 200 mg t.i.d. |
|
Zidovudine + |
Combivir |
One tablet p.o., b.i.d. |
|
lamivudine* |
||
|
Zidovudine + |
Trizivir |
One tablet p.o., b.i.d. |
|
lamivudine + |
||
|
abacavir* |
||
|
Nucleotide RTI |
||
|
Tenofovir |
Viread |
300 mg p.o., q.d. |
|
Nonnucleoside RTIs |
||
|
Delavirdine |
Rescriptor |
400 mg p.o., t.i.d. |
|
Efavirenz |
Sustiva |
600 mg p.o., q.d. (dose can be split and given 200 mg in A.M. and 400 mg in P.M.) |
|
Nevirapine |
Viramune |
200 mg p.o., q.d. x 14 days, then 200 mg p.o., b.i.d. |
|
Protease inhibitors |
||
|
Atazanavir |
Reyataz |
400 mg p.o., q.d. |
|
Fosamprenavir |
Lexiva |
1,400 mg q.d. or 700 mg b.i.d. |
|
Indinavir |
Crixivan |
800 mg p.o., q. 8 hr |
|
Lopinavir + |
Kaletra |
3 p.o., b.i.d. (lopinavir, 400 mg |
|
ritonavir |
b.i.d. + ritonavir, 100 mg b.i.d.) |
|
|
Nelfinavir |
Viracept |
1,250 mg p.o., b.i.d., or 750 mg p.o., t.i.d. |
|
Ritonavir |
Norvir |
600 mg p.o., b.i.d. (dose escalation1) |
|
Saquinavir |
Fortovase |
1,200 mg p.o., t.i.d. |
^Standard dosages are used in fixed combinations.
Ritonavir dose escalation: 300 mg p.o., b.i.d. x 2 days; 400 mg p.o., b.i.d. x 3 days; 500 mg p.o., b.i.d. x 8 days; 600 mg p.o., b.i.d. RTI — reverse transcriptase inhibitor
Figure 12 In this illustration, the drug levels of a protease inhibitor are compared with boosted levels of ritonavir. (IC90—concentration of drug required to inhibit 90% of viral replication)
Drug Selection
Adverse Effects of Drugs
Potential adverse effects can greatly influence the choice of an-tiretroviral medications and a patient’s ability and willingness to stay on long-term antiretroviral therapy [see Table 7]. Possible acute life-threatening reactions include didanosine-induced pancreatitis, abacavir-related hypersensitivity syndrome, lactic aci-dosis secondary to the use of any of the NRTIs but more closely associated with stavudine, Stevens-Johnson syndrome secondary to the use of any of the NNRTIs, and nevirapine-associated liver failure.97 Several older reports have described fatalities in patients taking hydroxyurea in combination with didanosine and with didanosine plus stavudine, so the use of hydroxyurea as adjuvant treatment with NRTIs is no longer recommended. Unfortunately, the pancreatitis associated with didanosine can occur without warning at any time during therapy.
A number of metabolic abnormalities have been reported in HIV-infected persons, some of which are linked to treatment with antiretroviral agents. Reported metabolic derangements include lactic acidosis, insulin resistance, hyperlipidemia, body-fat redistribution (lipodystrophy), osteonecrosis, and osteopenia.98
Hypersensitivity syndrome The abacavir hypersensitivity syndrome occurs in approximately 2% to 3% of patients receiving that drug and is strongly associated with a particular HLA haplotype (HLA-B*5701, HLA-DR7, and HLA-DQ3).99 It typically develops within the first 3 to 4 weeks after abacavir is started. Symptoms of abacavir hypersensitivity syndrome most often consist of rash, fever, nausea, oral lesions, and cough. Patients often describe an accentuation of these symptoms shortly after taking each dose of abacavir. Patients who begin to develop the hypersensitivity syndrome and then stop taking abacavir can have an acute, potentially fatal reaction on resuming abacavir therapy. Treatment of a patient with mild symptoms that are suggestive of abacavir hypersensitivity should be conducted in consultation with an expert on this matter.
Lactic acidemia Lactic acidemia is defined as an elevated venous lactate level (> 2 mmol/L) and a normal arterial pH. Lactic acidosis is present when the arterial pH is below 7.3. Mild, usually asymptomatic lactic acidemia occurs in 8% to 21% of patients on antiretroviral therapy,100,101 whereas symptomatic acide-mia occurs in less than 2.5%. The leading proposed mechanism is NRTI inhibition of the mitochondrial DNA gamma poly-merase. Risk factors for the development of lactic acidemia include longer duration of NRTI exposure (especially with stavu-dine102), female gender, and pregnancy. Symptoms associated with increased serum lactate levels are generally vague and may include nausea, malaise, and anorexia and weight loss, which may go on for months. Symptoms of more severe disease include abdominal pain, dyspnea, and encephalopathy. Although severe lactic acidosis is unusual, patients with serum lactate levels greater than 10 mmol/L have a mortality of approximately 80%.97 Treatment of symptomatic lactic acidemia should include discontinuance of NRTIs and supportive care. Most patients can be successfully rechallenged with other NRTIs after the acidemia has resolved.103
Table 5 Ritonavir Boosted Protease Inhibitor Regimens
|
Ritonavir Dose |
Other Protease Inhibitor |
Comment |
|
100 mg b.i.d. |
Lopinavir, 400 mg b.i.d. |
FDA-approved fixed combination with excellent potency |
|
400 mg b.i.d. |
Saquinavir, 400 mg b.i.d. |
Combination with best long-term data; highly effective combination, but intolerance to ritonavir common at this dosage Can use with hard-gel (Invirase) or soft-gel (Fortovase) formulations |
|
100 mg b.i.d. |
Saquinavir, 1,000 mg b.i.d. |
Can use with hard-gel (Invirase) or soft-gel (Fortovase) formulations, but better pharmaco-kinetic profile with hard-gel formulation |
|
100 mg b.id. or 200 mg b.i.d. |
Indinavir, 800 mg b.i.d. |
Moderate amount of data suggest regimen very potent; can be taken with meals; increased indinavir toxicity at this dosage |
|
400 mg b.i.d. |
Indinavir, 400 mg b.i.d. |
Moderate amount of data suggest regimen very potent; ideal pharmacokinetics, but intolerance to ritonavir common at this dosage |
|
200 mg q.d. |
Fosamprenavir, 1,400 mg q.d. |
Once-daily dosing of fosamprenavir not recommended for protease inhibitor-experienced patients |
|
100 mg b.i.d. |
Fosamprenavir, 700 mg b.i.d. |
Table 6 DHHS Recommendations for Antiretroviral Regimens for the Treatment of Established HIV in Adults and Adolescents87*
|
Regimen Type |
Drugs |
No. of Pills per Day |
|
NNRTI based |
Efavirenz + lamivudine + (zidovudine or tenofovir DF or stavudine*)+ |
3-5 |
|
Efavirenz + emtricitabine + (zidovudine or tenofovir DF or stavudine*)^ |
3-4 |
|
|
Favirenz + (lamivudine or emtricitabine) + (didanosine or abacavir) |
3-5 |
|
|
Nevirapine^ + (lamivudine or emtricitabine) + (zidovudine or stavudine* or didanosine or abacavir) |
4-5 |
|
|
PI based |
Lopinavir/ritonavir (Kaletra) + lamivudine + (zidovudine or stavudine*) |
8-10 |
|
Atazanavir + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir)+ |
4-5 |
|
|
Fosamprenavir + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir) |
6-8 |
|
|
Fosamprenavir/ritonavir§ + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir) |
6-8 |
|
|
Indinavir/ritonavir§ + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir) |
8-11 |
|
|
Lopinavir/ritonavir (Kaletra) + emitricitabine + (zidovudine or stavudine* or abacavir) |
8-9 |
|
|
Lopinavir/ritonavir (Kaletra) + lamivudine + abacavir |
8-9 |
|
|
Nelfinavir” + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir) |
12-14 |
|
|
Saquinavir (SGC or HGC)/ritonavir§ + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir) |
14-16 |
|
|
Triple NRTI1 |
Abacavir + lamivudine + (zidovudine or stavudine*) |
2-6 |
Note: Preferred regimens, drugs, and doses are in bold type: regimens are designated as preferred for use in treatment-naive patients when clinical trial data suggest optimal and durable efficacy with acceptable tolerability and ease of use. Alternative regimens are those for which clinical trial data show efficacy, but the preferred agent has better antiviral activity or demonstrated durable effect, tolerability, or ease of use. In some cases, a regimen listed as an alternative regimen in the table may actually be the preferred regimen because of individual patient characteristics.
*Higher incidences of lipoatrophy, hyperlipidemia, and mitochondrial toxicities are reported with stavudine than with other NRTIs. +Regimen not to be used in women with childbearing potential (i.e., women who want to conceive or who are not using effective contraception).
tA high incidence of symptomatic hepatic events has been observed in women with pre-nevirapine CD4+ T cell counts > 250 cells/^l (11%) and in men with counts > 400 cells/^l (6.3%). Use with caution in these patients, with close clinical and laboratory monitoring, especially during the first 18 wk of therapy. §Low-dose (100-200 mg) ritonavir. | Nelfinavir is available in a 250 mg or 625 mg tablet.
1A triple-NRTI regimen is to be used only when an NNRTI-based or a PI-based regimen cannot or should not be used as first-line therapy.
HGC—hard gel capsule
NRTI—nucleoside reverse transcriptase inhibitor
NNRTI—nonnucleoside reverse transcriptase inhibitor
PI—protease inhibitor
SGC—soft gel capsule
Insulin resistance Protease inhibitor-based regimens are associated with significant increases in insulin resistance (i.e., increased insulin concentrations and increased C-peptide levels) 104-106; in one historical cohort study of 221 patients, the reported incidence of new-onset hyperglycemia was 5%.107 Other retrospective studies have reported hyperglycemia in 3% to 17% of patients. Postulated mechanisms include a direct effect of HIV therapy on glucose uptake, drug-induced body-fat redistribution (lipodystrophy) (see below) that is associated with increased insulin resistance, and, in the case of indinavir,108 inhibition of peroxisome proliferator-activated receptor gamma. Reported treatment strategies include substitution of the protease inhibitor with an NNRTI or abacavir.109-111 Otherwise, treatment should be as recommended for non-HIV-infected patients with diabetes and should include diet and exercise, followed by insulin-sensitizing agents and insulin, if necessary.
Hyperlipidemia Before the widespread use of antiretroviral therapy, reduced levels of high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and total cholesterol and elevated levels of triglycerides were reported in HIV-in-fected patients.112-114 The use of protease inhibitors has been associated with a return of LDL cholesterol levels to normal or with high LDL cholesterol levels in conjunction with marked hypertriglyceridemia.115-118 Ritonavir has been associated with the greatest increases in triglyceride levels,117 whereas other protease inhibitors, such as atazanavir and nelfinavir, are less closely linked to this effect. The evidence of an association between NNRTIs and hyperlipidemia is mixed. Improvements in hyperlipidemia are more marked in patients changing from a protease inhibitor to nevirapine than to efavirenz.110,119,120 Except for one study showing higher lipid levels with stavudine than with tenofovir-DF, the NRTIs have not been associated with hyperlipidemia.121
The important clinical question is whether these abnormal lipid values translate into an increased risk of cardiovascular and cerebrovascular disease. Two very large studies reached different conclusions regarding this issue. A study that retrospectively abstracted coded data from records of 36,000 HIV-in-fected Veterans Affairs patients followed between 1995 and 2001 found a reduction in the rate of hospital admission for cardiovascular or cerebrovascular disease and a reduction in death from all causes that was associated with antiretroviral therapy.122 In contrast, a study of 23,000 HIV-infected patients enrolled in the multinational Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study demonstrated a 26% increase in the risk of myocardial infarction for each year of antiretroviral treat-ment.123 Neither study had the long follow-up periods usually needed to assess cardiovascular disease outcomes, and neither study included an HIV-negative control group. Nevertheless, it is likely that the hyperlipidemia seen in antiretroviral-treated HIV-infected patients is one of several factors that does increase the risk of development of cardiovascular and cerebrovascular disease. However, this potentially adverse effect is far outweighed by the benefits of antiretroviral therapy.
Table 7 Adverse Effects Associated with Antiretroviral Medications
|
Generic Name |
Trade Name |
Toxicity |
|
Nucleoside RTIs |
All nucleoside RTIs may be associated with mitochondrial toxicity, lactic acidemia, and lipodystrophy (atrophy) |
|
|
Abacavir (ABC) |
Ziagen |
Hypersensitivity reaction in 3% of patients; characterized by fever, rash, nausea, vomiting, or malaise; may be fatal; lactic acidosis (rare) |
|
Didanosine (ddI) |
Videx |
Peripheral neuropathy, pancreatitis, nausea, lactic acidosis, lipoatrophy |
|
Emtricitabine (FTC) |
Emtriva |
Few side effects; lactic acidosis (rare) |
|
Lamivudine (3TC) |
Epivir |
Few side effects; lactic acidosis (rare) |
|
Stavudine (d4T) |
Zerit |
Peripheral neuropathy; lactic acidosis (rare) |
|
Zalcitabine (ddC) |
Hivid |
Peripheral neuropathy, stomatitis, lactic acidosis (rare) |
|
Zidovudine (AZT) |
Retrovir |
Anemia, neutropenia, gastrointestinal problems, headache, lactic acidosis (rare), lipoatrophy |
|
Nucleotide RTIs Tenofovir |
Viread |
Renal dysfunction (unusual) |
|
Nonnucleoside RTIs Delavirdine |
Rescriptor |
Rash (4%); increased transaminase levels; headache |
|
Efavirenz |
Sustiva |
Rash seen in 2% of patients; central nervous system side effects (confusion, abnormal dreams, agitation, etc.) |
|
Nevirapine |
Viramune |
Rash seen in 7% of patients; increased transaminase levels; hepatitis (can be fatal) |
|
Protease inhibitors |
Most protease inhibitors are associated with lipid abnormalities, lipodystrophy (central fat accumulation) and gastrointestinal side effects |
|
|
Fosamprenavir Atazanavir |
Lexiva Reyataz |
Gastrointestinal intolerance; lipid abnormalities; perioral paresthesias; fat redistribution Gastrointestinal intolerance, elevated bilirubin levels |
|
Indinavir |
Crixivan |
Gastrointestinal intolerance; nephrolithiasis; increase of indirect bilirubin; headache; lipid abnormalities; fat redistribution |
|
Lopinavir + ritonavir |
Kaletra |
Gastrointestinal intolerance; lipid abnormalities, including increased triglyceride levels; fat redistribution |
|
Nelfinavir |
Viracept |
Diarrhea; lipid abnormalities; major drug-drug interactions; fat redistribution |
|
Ritonavir |
Norvir |
Gastrointestinal intolerance; perioral and extremity paresthesias; taste perversions; asthenia; hepatitis; major drug-drug interactions; lipid abnormalities, including increased triglyceride levels; fat redistribution |
|
Saquinavir |
Fortovase, Invirase |
Gastrointestinal intolerance; headache; lipid abnormalities; fat redistribution |
RTI — reverse transcriptase inhibitor
Current recommendations from an International AIDS Society-USA panel for treatment of hyperlipidemia include the following: (1) a possible change to protease inhibitor-sparing antiretroviral regimens, (2) adherence to diet and exercise guidelines, and (3) use of lipid-lowering agents.98 Specific recommendations for the use of lipid-lowering agents follow those of the United States National Cholesterol Education Program guide-lines.124 Of the statins, pravastatin has the least severe drug interactions with protease inhibitors and is often used as a first-line agent. Although atorvastatin has more interactions with protease inhibitors than does pravastatin, few adverse events have been reported with atorvastatin. In addition, atorvastatin has greater potency than pravastatin, and it has a favorable impact on triglyceride levels.125 Patients with marked hypertriglyc-eridemia often require treatment with a fibric acid analogue (e.g., fenofibrate or gemfibrozil).
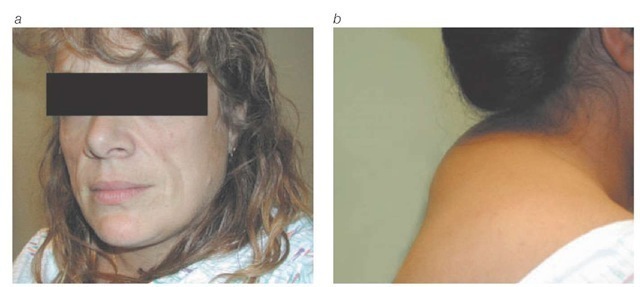
Lipodystrophy The lipodystrophy syndrome, also known as the fat-redistribution syndrome, has become a problematic long-term complication of antiretroviral therapy. The lipodys-trophy syndrome typically presents as central fat accumulation (i.e., accumulation of fat in the abdomen, breasts, or posterior neck) or peripheral fat wasting (i.e., wasting in the face, buttocks, and limbs), or both126 [see Figure 13]. Lipodystrophy is reported in 25% to 75% of HIV-infected patients.
Several mechanisms have been proposed for the lipodystro-phy syndrome, but none are proven. Protease inhibitors, because of their role in inducing insulin resistance, have been blamed for the central lipoaccumulation.112,130 Fat accumulation has also been reported in HIV-infected patients who were not exposed to protease inhibitors, however.127,131 Lipoatrophy is more closely tied to exposure to NRTIs,126,132,133 particularly stavu-dine. In this setting, NRTI-induced mitochondrial poisoning (as occurs in NRTI-induced lactic acidemia) may be responsible for adipocyte death and loss of subcutaneous fat. Other possible risk factors for the syndrome include older age, low body weight before starting antiretroviral therapy, duration of HIV infection, duration and effectiveness of antiretroviral therapy, and white race.
A number of drugs and interventions have been tried as treatment for lipodystrophy, with only modest success. Switching from stavudine to other NRTIs has reversed limb-fat atrophy in some patients,137 but substitution of protease inhibitors to reverse fat accumulation has not been successful. Exercise may have only a limited benefit in reducing fat accumulation,138 but it does have other obvious health advantages. Growth hormone has been shown to benefit a small number of patients with fat accu-mulation,139 but this approach is not considered practical, given the extreme cost, adverse effects, and the need for continued use to sustain benefit. Promising results in the treatment of lipodys-trophy have been reported from preliminary studies of the insulin-sensitizing agent metformin. In a randomized, double-blind, placebo-controlled trial, use of metformin at a dosage of 500 mg twice daily for 3 months was associated with decreased insulin levels, decreased weight, decreased visceral abdominal fat, and decreased subcutaneous abdominal fat.140 In this study, patients taking metformin did not experience an increase in serum lactate levels or hepatic transaminase levels. Similarly, a study of the insulin sensitizer rosiglitazone showed that this agent can improve insulin sensitivity, reduce free fatty acid levels, and increase subcutaneous leg fat in patients with lipodys-trophy.141 No change was seen in subcutaneous abdominal or visceral fat. The study was small (28 patients) and of short duration (3 months) but was randomized, double blind, and placebo controlled and will hopefully be confirmed by larger studies with longer follow-up. These reports provide further indirect evidence that insulin resistance plays a central role in the patho-genesis of HIV-associated lipodystrophy. Further study is needed to better predict which patients will develop fat-redistribution syndrome and to identify preventive measures.
Figure 13 Typical presentations of the lipodystrophy syndrome include (a) wasting of facial fat and (b) accumulation of fat in the posterior neck.
Bone abnormalities Avascular necrosis (AVN), osteopenia, and osteoporosis are becoming prominent complications of HIV infection and may be linked to antiretroviral therapy. AVN is still an unusual clinical diagnosis, although one study using magnetic resonance imaging scanning documented occult AVN of the femoral head in 5% of HIV-infected patients.142 Traditional risk factors for AVN include trauma, systemic lupus erythema-tosus, corticosteroid use, hemoglobinopathies, hypercoagulable states, hyperlipidemia, and alcohol abuse, whereas case-control studies involving HIV-infected patients have demonstrated links only to steroid use and hyperlipidemia.142-144 HIV-associat-ed AVN occurs more frequently in the hips and knees and is often bilateral, distinguishing it from AVN in HIV-negative persons, which is usually not multifocal. Treatment options for AVN are limited to pain control and, in severe cases, surgery.
Reports of decreased bone mineral density in HIV-infected patients predate the use of potent antiretroviral therapy.145 However, studies have demonstrated an association between protease inhibitors, osteopenia, and lipodystrophy.146,147 The patho-genesis of HIV-associated bone abnormalities is unknown. Regular screening for osteopenia in HIV-infected patients is not currently recommended. If patients are discovered to have decreased bone mineral density, treatment should include calcium and vitamin D supplementation. Frank osteoporosis may merit the addition of bisphosphonates, calcitonin, or estrogen receptor modulators.