Membranous Nephropathy
Although membranous nephropathy derives its name from the characteristic thickening of the GBM seen on light microscopy, the primary site of injury is the podocyte. In turn, podocyte dysfunction results in increased extracellular matrix protein accumulation, causing the basement membrane thickening that is the hallmark of the disease.
Membranous nephropathy is the most common cause of the nephrotic syndrome in white adults and is the leading cause of nephrotic syndrome in persons older than 60 years. In adults, 80% of cases are idiopathic; the remaining 20% are from secondary causes [see Table 4]. The most common secondary causes are SLE; hepatitis B infection66; drugs (e.g., gold, penicil-lamine); and, in the elderly, solid tumors—malignancy underlies 20% of cases in patients older than 50 years.67 Membranous nephropathy is typically a disease of men (the male-to-female ratio is 2:1 to 3:1) and has a biphasic age distribution of 30 to 40 years and 50 to 60 years.
Pathogenesis and histology Although thickening of the GBM characterizes membranous nephropathy, it occurs late in the course of disease. The pathognomonic finding is diffuse deposition of finely granular immune deposits at the base of the podocytes on the outer surface of the GBM, which are referred to as subepithelial or subpodocyte immune deposits [see Figure 14]. The location of the immune deposits and evidence from experimental models of disease suggest that the target of disease is one or more antigens on the podocytes.68 The inciting antigen (or antigens) remains to be identified in humans. However, the concurrent presence of subendothelial and mesangial deposits is more suggestive of circulating antigens or antigen-antibody complexes. Antibodies such as antineuroendopepti-dase have been linked with the onset of membranous nephropathy. Membranous nephropathy is mediated by a humoral immune response, leading to deposition of IgG and C3, but not C4, indicating activation of the alternative complement pathway. Immunostaining for C5b-9 (the membrane attack complex) occurs in a pattern similar to that for IgG [see Figure 15]. Consequences of C5b-9 injury to podocytes include production of oxidants, cytokines, proteases, and growth factors that further damage the podocyte and the underlying GBM. The presence of C1q on the biopsy suggests lupus nephritis class V as the secondary cause.
Later in the course of disease, thickening of the GBM is evident on light microscopy. Silver immunostaining, which is used to detect organized matrix, shows the characteristic spike pattern of membrane thickening forming around the subepithelial deposits. Electron microscopy shows flattening, fusion, and efface-ment of the podocytes, which augments proteinuria. As in most forms of progressive glomerular disease, further deterioration in renal function is caused by progressive tubulointerstitial fibrosis.
Clinical and laboratory findings Patients with membranous nephropathy present with the complications of marked nephrotic-range proteinuria (> 3.5 g/day), including edema and fluid retention. The proteinuria is nonselective (i.e., including not only albumin but also larger proteins) and is usually in the range of 5 to 15 g/day. However, 20% of patients with membranous nephropathy have non-nephrotic-range proteinuria and therefore may be asymptomatic. Clinical clues to secondary causes may be evident at presentation in patients who have systemic symptoms (e.g., a rash in patients with SLE). Microscopic hema-turia is noted in 50% of patients, yet the urine is devoid of RBC casts and WBCs. Because the decline in GFR occurs slowly in membranous nephropathy, renal function is normal in 80% of patients at presentation. Hypertension occurs in 30% of cases.
|
Table 4 Conditions Associated with Membranous Nephropathy |
|
Tumors (e.g., bowel, breast, lung) |
|
Infections (e.g., hepatitis B, hepatitis C, syphilis, filariasis, hydatid disease, schistosomiasis, malaria, leprosy) |
|
Drugs and toxins (e.g., gold, penicillamine, nonsteroidal anti-inflammatory drugs, mercury, captopril, formaldehyde, hydrocarbons) |
|
Immune diseases (e.g., systemic lupus erythematosus, diabetes mellitus, rheumatoid arthritis, Hashimoto disease, Graves disease, mixed connective tissue disease, Sjogren syndrome, primary biliary cirrhosis, bullous pemphigoid, small bowel enteropa-thy syndrome, dermatitis herpetiformis, ankylosing spondylitis, graft versus host disease, Guillain-Barre syndrome) |
|
Miscellaneous conditions (e.g., sarcoidosis, Kimura disease [angio-lymphoid hyperplasia with eosinophilia], angiofollicular lymph node hyperplasia) |
Figure 14 Transmission electron micrograph showing characteristic subepithelial cell deposits (arrows) in (a) early and (b) late membranous nephropathy; P illustrates podocytes, and L shows the capillary lumen.
Positive laboratory studies in membranous nephropathy include those providing evidence of nephrotic syndrome (i.e., hy-poalbuminemia, hypercholesterolemia, and proteinuria). Although hypercoagulability can complicate any form of the nephrotic syndrome, renal vein thrombosis is most commonly found in membranous nephropathy and may occur in up to 50% of patients. Despite the local activation of complement in the glomerulus, serum total complement and individual complement components are normal in idiopathic membranous nephropathy. In contrast, low complement levels support secondary causes, such as SLE, hepatitis B, and hepatitis C. Investigations for specific secondary causes should be sought, especially in patients older than 50 years.
Treatment and prognosis Management of membranous nephropathy includes the treatment of the underlying disease in secondary forms of membranous nephropathy and prevention or reduction of complications of nephrotic syndrome. The prognosis of idiopathic membranous nephropathy has traditionally been known by the rule of thirds69: about one third of patients have a spontaneous, complete remission within 3 to 5 years, with resolution of proteinuria and normalization of the GFR; one third have partial remission, with persistent proteinuria of less than 2 g/day but a normal GFR; and one third progress to end-stage renal disease. Some studies have shown that closer to 50% of patients progress to end stage. In these patients, GFR drops 50% over 3 to 4 years. Poor prognostic markers include male sex, age over 60 years, hypertension, proteinuria that is massive in both quantity and duration (e.g., > 8 g for 6 months, > 6 g for 9 months, or > 4 g for 12 months), and a decrease in GFR.
Figure 15 (a) Silver staining showing thickened GBM and spike formation in membranous nephropathy. (b) Immunofluorescent staining showing the typical granular pattern of IgG in membranous nephropathy. (c) C5b-9 immunostaining in a pattern similar to that of IgG.
Renal injury can be reduced by controlling hypertension and lowering proteinuria or by the judicious use of ACE inhibitors, ARBs, or both. In patients who are at high risk for progressive re-nal failure, disease-specific treatment should be implemented in addition to these nonspecific measures [see Figure 16].70
Figure 16 Treatment algorithm for high-risk patients with membranous nephropathy.
In contrast to idiopathic FSGS and minimal change disease, membranous nephropathy cannot be adequately treated with corticosteroids alone; instead, disease-specific therapy includes both alkylating agents and steroids. Two regimens are used. The first is oral cyclophosphamide (1 to 2 mg/kg/day) with prednisone (0.5 mg/kg/day) for 6 months.71-73 A second, proposed protocol involves alternating cycles of oral chlorambucil (0.1 to 0.2 mg/kg/day) for 1 month and steroids for 1 month (methyl-prednisolone, 1g I.V., followed by 0.4 to 0.5 mg/kg/day of oral prednisone for 27 days).74
If alkylating agents are contraindicated or if the patient experiences relapses, the second line of therapy is oral cyclosporine (3.5 to 5.0 mg/kg/day) for 1 to 2 years.75 Alternative and experimental therapies include intravenous immunoglobulin and my-cophenolate mofetil and, more recently, rituximab. These therapies should be administered by a nephrologist. Renal transplantation for patients with end-stage renal failure from membranous nephropathy is encouraged, although there is a slight (5%) risk of subsequent occurrence in the donor kidney.
Minimal Change Disease
Minimal change disease derives its name from the lack of clear-cut histologic abnormalities visible on light microscopy of renal biopsy specimens.76 Minimal change disease is the most common form of primary glomerular disease causing nephrotic syndrome in children and is the third most common cause of nephrotic syndrome in adults.77 There is no gender predilection in adults, whereas twice as many boys as girls are affected.
Although most forms of minimal change disease are idiopath-ic, secondary causes need to be ruled out [see Table 5]. A combination of the nephrotic syndrome and renal failure should alert the clinician to NSAID-induced minimal change disease complicated by interstitial nephritis.
|
Table 5 Causes of Minimal Change Disease |
|
Drugs (e.g., nonsteroidal anti-inflammatory drugs, interferon alfa, lithium, gold) |
|
Allergies (e.g., pollen, house dust, insect stings, immunizations) |
|
Malignancies (e.g., Hodgkin lymphoma, leukemia) |
Pathogenesis and histology Minimal change disease is probably caused by a defect in cell-mediated immunity, particularly T cells. Podocytes are the target of injury. However, no abnormalities are seen on light microscopy, and immunofluores-cent staining is negative for immunoglobulins and complement factors. On electron microscopy, the classic finding is flattening, effacement, and fusion of the foot processes.80 Podocyte injury leads to a decrease in production of negatively charged proteo-glycans in the underlying basement membrane and foot processes, which underlies the development of proteinuria.
Clinical and laboratory findings Because the onset of pro-teinuria is typically abrupt in minimal change disease, patients present with acute clinical findings of the nephrotic syndrome. The laboratory abnormalities usually reflect the severity of the nephrotic syndrome. Hypertension and a decline in renal function occur in fewer than 20% of affected adults. Proteinuria is typically massive (> 6 g/day), urine sediment is unremarkable, and hematuria is rare.
*Heavy proteinuria for more than 12-16 wk.
Figure 17 Treatment algorithm for patients with minimal change disease.
Table 6 Causes of Membranoproliferative Glomerulonephritis Types I-III
|
Type |
Causes |
|
I |
Idiopathic |
|
Collagen vascular diseases (e.g., rheumatoid arthritis, Sjogren syndrome, systemic lupus erythematosus) |
|
|
Complement deficiencies |
|
|
Acquired (e.g., C4 nephritic factor) Hereditary (e.g., C1q, C2, C3, C4) |
|
|
Cryoglobulinemia (types 2 and 3) Infections |
|
|
Viral (e.g., hepatitis C, chronic hepatitis B, HIV) Bacterial (e.g., infective endocarditis, visceral abscess, infected shunt) |
|
|
Other (e.g., Plasmodium malariae, Schistosoma, Mycoplasma) |
|
|
Malignancies (e.g., chronic lymphocytic leukemia, lymphoma, renal cell carcinoma) |
|
|
Other (e.g., ^-antitrypsin deficiency, other chronic liver diseases, hypocomplementemic urticaria vasculitis) |
|
|
II |
Idiopathic |
|
Associated with C3 nephritic factor Associated with factor H defect |
|
|
Partial lipodystrophy |
|
|
III |
Idiopathic |
|
Associated with terminal complement nephritic factor |
|
|
Same causes listed for type I (see above) |
Treatment and prognosis Corticosteroids are the treatment of choice for minimal change disease [see Figure 17]. In adults, prednisone (1 mg/kg/day) should be given for 8 to 16 weeks. In those patients who respond (> 95%), prednisone is then switched to alternate days at a dosage of 1 mg/kg for 4 weeks, followed by tapering over the next few months.80 A first relapse should be treated with another course of steroids. In patients who have relapses more than twice after completion of the steroid course and in patients who have a relapse during the taper (i.e., steroid-dependent patients), cyclophosphamide (2 mg/kg/day) should be given for 8 to 12 weeks, combined with prednisone at an increased dosage. Alternatives to cytotoxic therapy include cyclosporine (4 to 6 mg/kg/day) along with alternate-day prednisone. Limited published experience with mycophenolate mofetil suggests some benefit in patients with refractory or relapsing disease.81-83 In patients who do not respond to prednisone within 12 to 16 weeks (i.e., steroid-resistant patients), cyclophosphamide or cyclosporine may be used. Levamasole, an immune stimulant, has also been used with some success in steroid-dependent children with nephritic syndrome from minimal change disease.84 In children who have frequent relapses after treatment with alkylating agents, lev-amisole may serve as an alternative.
Diseases presenting as mixed nephritic-nephrotic syndrome
Membranoproliferative Glomerulonephritis
MPGN, which is also known as mesangiocapillary glomeru-lonephritis, is an immune complex-mediated glomerular dis-ease.86 It can present as nephritic, nephrotic, or nephritic-nephrotic syndrome. The name derives from the classic finding of splitting of the basement membrane and proliferation of mesangial and endothelial cells. MPGN accounts for 5% to 20% of all primary nephrotic syndromes. The incidence of the disease is highest in South America and Africa, and it occurs equally in males and females. MPGN is mostly idiopathic, but the histolog-ic diagnosis should always prompt a search for secondary causes [see Table 6].
Pathogenesis and histology MPGN is classified into types I, II, and III, according to the sites of deposition of glomerular immune complexes (IgG and C3). These are preformed immune complexes, such as hepatitis C antigen and anti-hepatitis C antibody. Each type of MPGN has a different cause and involves activation of different components of the complement pathway, giving rise to differences in serum complement levels.
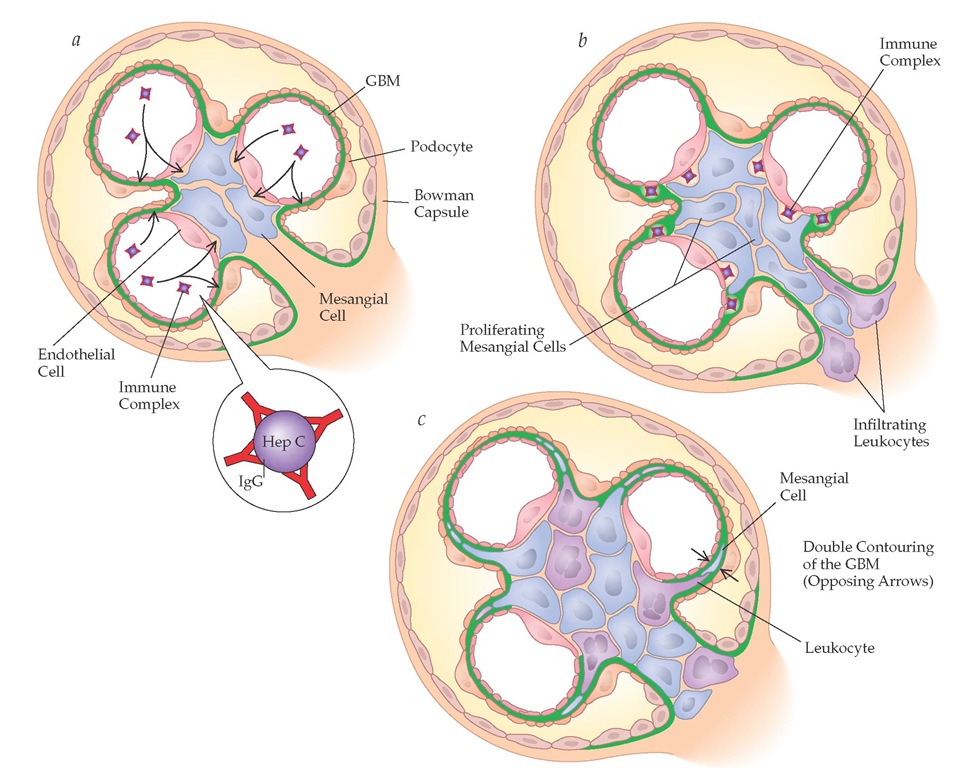
MPGN type I refers to immune complex deposits that are subendothelial and mesangial and result from diseases characterized by chronic immune complex formation that activates the classic complement pathway [see Figure 18].88 The most common cause of MPGN type I is hepatitis C, with or without mixed cryo-globulinemia.26 Less frequent causes include mixed cryoglobu-linemia that does not result from hepatitis C infection, hepatitis B infection, or subacute bacterial endocarditis. The classic double contouring (so-called tram tracks) seen on histology is a result of interposition of mesangial cells, leukocytes, or endothelial cells in the capillary wall, with the synthesis of new basement membrane material. Resident mesangial and endothelial cells and infiltrating cells proliferate in MPGN. Immunofluorescent staining is positive for IgG, IgM, and C3 along the capillary wall; these immune complexes take on a granular pattern, which is distinct from the linear pattern in anti-GBM disease.
MPGN type II occurs primarily in young adults and children. It is caused by activation of the alternative complement pathway and is associated with the presence of dense deposits in the basement membrane itself that are positive for C3 but negative for IgG and for the classic complement components C1q and C4.89 Accordingly, type II MPGN is also called dense-deposit disease. Its etiology is unknown, but possible causes include the absence of a constitutive inhibitor of the alternative complement pathway (factor H) or the presence of a circulating autoantibody (C3 nephritic factor) that binds to and prevents inactivation of the alternative pathway C3 convertase.
MPGN type III has lesions that are similar to those of type I but have additional subepithelial deposits. Occasionally, in-tramembranous deposits are also present.
Clinical and laboratory findings MPGN has four clinical presentations: microscopic hematuria and nonnephrotic protein-uria, which is seen in 35% of patients; nephrotic syndrome and a mild decrease in renal function, seen in 35% of patients; chronic glomerulonephritis, usually progressive, in 20% of patients; and acute renal failure with proteinuria and an active urine sediment with RBC casts, in 10%. Hypertension is a very common presenting finding, occurring in 50% to 80% of patients, and the urinary sediment is often active. One quarter of patients with MPGN type II may also manifest partial lipodystrophy that preferentially involves the face and upper body.
Although patients present with evidence of glomerular disease, clues to a secondary cause may also be present, such as features of cryoglobulinemia (e.g., weakness, arthralgia, purpura), viral infection (e.g., hepatitis B, hepatitis C, HIV), bacterial infection (e.g., endocarditis, abscess), and liver disease. Laboratory evidence for MPGN can be categorized as follows: the presence of the nephrotic syndrome or renal failure, or both, and the complications thereof; activation of the classic complement cascade, with low levels of C1q, C4, and CH50, which is seen in MPGN types I and III; and activation of the alternative pathway, with low levels of C3 and CH50 and normal levels of C1q and C4, which is characteristic of MPGN type II. In addition, the clinician should look for evidence of an underlying cause of MPGN, such as the presence of circulating immune complexes, cryoglobulins, rheumatoid factor, and specific viruses (e.g., hepatitis C virus RNA, hepatitis B markers, HIV).
Figure 18 The pathogenesis of membrano-proliferative glomerulonephritis (MPGN) type I associated with hepatitis C infection. (a) Circulating immune complexes consisting of hepatitis C antigen and anti-hepatitis C antibody enter the endothelium, resulting in (b) proliferation of mesangial cells and infiltration of leukocytes. (c) Interposition of mesangial cells and leukocytes leads to double contouring of the GBM.
Treatment and prognosis Hepatitis C-associated MPGN with cryoglobulinemia is usually treated with antiviral therapy (i.e., PEG-interferon alpha-2b and ribavirin, if renal function allows). Remission occurs in 60% of patients, but more than 80% of patients experience relapses. Other infection-associated MPGN usually responds to specific antimicrobial therapy, which reduces the antigen levels and therefore the immune complexes. Persistent signs of renal disease and hypocomplementemia usually indicate inadequate therapy. Steroids have been administered on alternate days in children with MPGN, but the use of steroids remains controversial in adults. On the basis of pediatric studies, long-term low-dose prednisone may be tried in adults with nephrotic-range pro-teinuria. For MPGN associated with rapidly progressive renal failure, combination therapy with steroids and cytotoxic agents is frequently used.92
The prognosis of MPGN depends in part on the presence of any underlying disease, which must be treated with specific therapy. Idiopathic MPGN has a poor prognosis: 40% to 50% of patients progress to renal failure within 10 years. Optimal therapy has not been established. In adults, studies have shown potential benefit with antiplatelet therapy such as aspirin (975 mg/day) and dipyridamole (225 mg/day), anticoagulation therapy such as warfarin, and steroids. Controlled trials of alkylating agents have not shown long-term benefit, and the therapeutic benefit of cyclosporine has not been adequately proved. MPGN recurs in renal transplant patients with type I disease (20% to 50% of patients) and type II disease (80% of patients) but is usually milder than in native kidneys.