Diagnosis
Nocturnal sleep symptoms of OSAS are loud snoring (often with a long history), choking, cessation of breathing, sitting up and fighting for breath, abnormal motor activities and thrashing about in bed, gastroesophageal reflux, nocturia and nocturnal en-uresis (seen mostly in children), and, occasionally, hyperhidrosis.
|
Table 3 Conditions Comorbid with Insomnia |
|
Psychiatric disorders |
|
Mood disorders (e.g., major depressive and bipolar disorders) |
|
Anxiety disorders (e.g., panic and posttraumatic stress disorder) |
|
Stress |
|
Other psychophysiologic factors |
|
Medical disorders |
|
Bronchial asthma |
|
Coronary artery disease |
|
Peptic ulcer disease |
|
Rheumatic disorders |
|
Neurologic disorders |
|
Stroke |
|
Neurodegenerative diseases |
|
Brain tumors Headache syndromes |
|
Neuromuscular disorders (e.g., painful peripheral neuropathies) |
|
Traumatic brain injury |
|
Fatal familial insomnia (a rare prion disease) |
|
Pain anywhere in the body |
|
Sleep-wake schedule disruptions |
|
Other disorders of circadian sleep rhythms |
|
Drug or alcohol abuse |
The major daytime symptom of OSAS is EDS; patients fall asleep during the day at inappropriate times and in inappropriate places and may be involved in driving accidents. They cannot function adequately during the day and may complain of morning headaches and forgetfulness; men may report impotence. The prolonged duration and the nonrefreshing nature of daytime sleep in EDS differentiate it from narcoleptic sleep attacks. Physical examination reveals obesity in about 70% of patients; in many cases, local anatomic abnormalities of the upper airway are found. Repeated hypoxemia associated with cessation of breathing during sleep can lead to hypertension, cardiac arrhythmias, heart failure, and stroke.23
Treatment
The general treatment of OSAS consists of avoiding sedatives, hypnotics, and alcohol and, in obese patients, reduction of body weight. Mild cases of OSAS may improve with these measures. Pharmacologic treatment remains unsatisfactory.
Application of CPAP is effective in over 70% of moderate to severe cases of OSAS and is the best treatment available.24 CPAP opens up the upper airway passages and acts as a pneumatic splint, thereby eliminating obstructive apneas, hypoxemias, snoring, and sleep fragmentation. The optimal pressure of CPAP must first be determined during overnight PSG. The patient can then purchase a home unit to use during nightly sleep. Manufacturers have devised auto-CPAP machines, which automatically titrate pressure according to detected problems. The role of these devices remains to be determined.
In the few severe cases of OSAS in which CPAP therapy fails, uvulopalatopharyngoplasty or other surgical approaches may be needed, although their roles remain uncertain. Laser or ra-diofrequency uvulopalatopharyngoplasty has been tried. Severe respiratory compromise or severe apnea associated with dangerous cardiac arrhythmias creates a life-threatening situation that may require emergency tracheostomy. In patients with neuro-muscular disorders, intermittent positive pressure ventilation through a nasal mask may be needed to treat sleep hypoventila-tion or apnea. In some mild to moderate cases of OSAS, dental appliances may be tried.
Narcolepsy
The most important clinical manifestations of narcolepsy are sleep attacks and cataplexy, although the ICSD-2 includes one category as narcolepsy without cataplexy.17,25 Narcoleptic sleep attacks usually begin in patients between 15 and 25 years of age but may occur earlier or later. Narcolepsy is a lifelong disorder, but it becomes less severe with old age. From 1% to 2% of the first-degree relatives of narcoleptic patients manifest the illness, compared with 0.02% to 0.18% in the general population.26 The cause of narcolepsy is unknown and is thought to result from an interplay of genetic and environmental factors. There is a strong association between narcolepsy and the presence of the HLA DQB1*0602 subtype.
The most exciting recent development in our understanding of narcolepsy is the documentation of an abnormality in the hypocretin (orexin) neurons in the lateral hypothalamus.27-32 Human narcolepsy-cataplexy can be considered a hypocretin (orex-in) deficiency syndrome. Four lines of evidence can be cited in support of hypocretin abnormality in narcolepsy: (1) induction of narcolepsy-like symptoms after mutation of the hypocretin receptor 2 gene in dogs and after preprohypocretin knockout in mice, (2) decreased hypocretin 1 levels in the cerebrospinal fluid of narcolepsy-cataplexy patients, (3) postmortem documentation of decreased numbers of hypocretin neurons in narcoleptic brains, and (4) preprohypocretin gene mutation in a child with severe narcolepsy associated with generalized absence of hypocretin peptides in the brain.
Diagnosis
The classic narcoleptic sleep attack consists of an irresistible desire to sleep in an inappropriate place and under inappropriate circumstances. Attacks generally last from a few minutes to 30 minutes, and the patient feels refreshed on awakening. The initial manifestations of narcolepsy are generally sleep attacks; after months or years, 60% to 70% of patients experience cata-plexy, during which they transiently lose muscle tone (e.g., sagging of the head, drooping of the eyelids, and buckling of the knees). Cataplexy is often triggered by a sudden emotional outburst. The patient may momentarily collapse and fall to the ground or may simply slump forward for a few seconds before regaining awareness.
Additional manifestations of narcolepsy include hypnagogic or hypnopompic hallucinations (fearful or vivid dreams at sleep onset or end), sleep paralysis (feeling of loss of power or focal weakness on one side of the body or in one limb during the transition period between sleep and wakefulness), disturbance of nocturnal sleep, and automatic behaviors (during which the patient might keep doing one thing repeatedly or drive to a place without recollection of the events). In some patients, narcoleptic sleep attacks may be associated with sleep apnea, REM behavior disorder, or PLMS.
Treatment
The administration of a stimulant (e.g., modafinil, methyl-phenidate, dextroamphetamine, or methamphetamine) is the treatment of choice for narcoleptic sleep attacks.25 In newly diagnosed patients, the drug most commonly used initially is moda-finil, a novel wakefulness-promoting agent, or methylphenidate. Dextroamphetamine and methamphetamine are used in patients who do not respond satisfactorily to these stimulant drugs.
Tricyclic antidepressants (e.g., protriptyline, imipramine, and clomipramine) and selective serotonin reuptake inhibitors (SSRIs, such as fluoxetine) are used to treat cataplexy or other auxiliary symptoms of narcolepsy. Sodium oxybate, which is more commonly known as y-hydroxybutyric acid (GHB) [see 8:I Management of Poisoning and Drug Overdose], has been approved by the Food and Drug Administration for the treatment of cata-plexy. It is an endogenous hypnotic that acts by consolidating REM and slow wave sleep. The medication is administered in two divided doses at night. The first dose is taken at bedtime; the patient must awaken 2.5 to 4 hours later to take the second dose. Taking short daytime naps and joining narcolepsy support groups can be useful approaches as well.
Idiopathic hypersomnia
The International Classification of Sleep Disorders defines id-iopathic hypersomnia as a disorder of excessive sleepiness of presumed CNS cause that is associated with major sleep episodes.17 The disorder is further categorized as with or without long sleep time (over 10 hours or from 6 to 10 hours, respectively). The disease develops insidiously, generally between the ages of 15 and 30 years. It closely resembles narcolepsy. Affected patients generally sleep for hours, and the sleep is not refreshing. The patient does not give a history of cataplexy or snoring.
Idiopathic hypersomnia is a disabling and lifelong disorder. The MSLT shows a mean sleep latency of less than 8 minutes without sleep-onset REMs. The treatment of idiopathic hyper-somnia is similar to the stimulant treatment of narcolepsy; however, the therapeutic response is unsatisfactory.
Restless legs syndrome and periodic limb movements in sleep
Restless Legs Syndrome
RLS is a lifelong sensorimotor neurologic disorder that may begin at any age.33 RLS is most severe in middle-aged or elderly persons, in whom it has a chronic, progressive course. The prevalence of RLS has been estimated to be about 10% for all adult populations, particularly those of European descent. However, in some surveys from Asia, the prevalence is extremely low, suggesting a possible ethnic and racial difference in RLS prevalence.
Clinical features Minimal criteria for the clinical diagnosis of RLS include the following four sensorimotor features33,34: (1) the patient has an urge or compulsion to move the legs, usually accompanied by uncomfortable or unpleasant sensations, primarily in the legs (generally below the knees) but sometimes in the arms; (2) the urge to move or uncomfortable sensations begin or worsen during rest and repose or inactivity; (3) the urge to move or uncomfortable sensations are partially or totally relieved by movement, at least in the beginning of the illness, and at least as long as the activity continues; (4) the uncomfortable sensations or the urge to move is worse in the evening or early part of the night than during the day.
Supportive diagnostic criteria include responsiveness to dopaminergic drugs, at least early in the disease; presence of periodic limb movements; and positive family history.34 Up to about 50% of patients have a family history of a similar condition, suggesting a dominant mode of inheritance. At least 80% of RLS patients have PLMS (see below). In addition, many RLS patients also have periodic limb movements in wakefulness (PLMW). Associated features of RLS include a progressive clinical course, normal neurologic examination in the idiopathic or primary type of RLS, and sleep disturbance.34
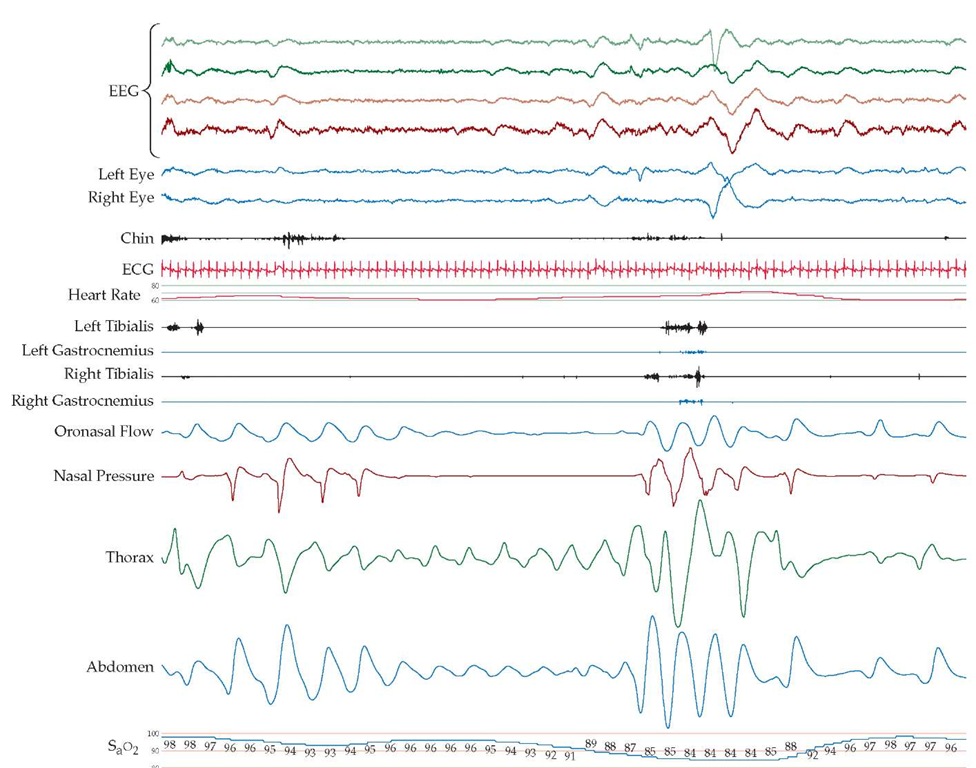
Figure 2 Polysomnographic (PSG) recording of a patient with sleep apnea syndrome includes EEG tracings (top four channels), EOG readouts of the left and the right eyes, EMG of mentalis muscle (chin), electrocardiogram, EMG of the left tibialis and right tibialis and gastrocnemius muscles, and recordings of oronasal airflow, nasal pressure, respiratory effort (thoracic and abdominal readings), and oxygen saturation (SaO2). The PSG study shows episodes of obstructive sleep apnea during stage II NREM sleep, followed by arousals.
Figure 3 Polysomnographic recording of a patient with restless legs syndrome includes EEG tracings (top four channels), EOG readouts of the left and the right eyes, EMG of the mentalis muscle (chin), electrocardiogram, EMG of the left and the right tibialis and gastrocnemius muscles, and recordings of oronasal airflow, nasal pressure, respiratory effort (thoracic and abdominal readings), and oxygen saturation (SaO2). The tibialis and gastrocnemius EMG recordings reveal periodic limb movements in sleep.
It is important to exclude potential underlying causes of RLS (e.g., iron deficiency anemia, uremia, and polyneuropa-thy). The condition may have a profound impact on sleep, and occasionally, patients have sleep apnea or daytime hy-persomnolence.
Periodic Limb Movements in Sleep
PLMS is common in the general population, with increasing prevalence in persons of advanced age; patients may remain completely asymptomatic.35,36 PLMS is a polysomnographic finding and is characterized by repetitive, often stereotyped limb movements during NREM sleep. The characteristic movements are usually extension of the great toe, dorsiflexion of the ankle, and flexion of the knee. Similar movements are sometimes seen in the arms. At least four consecutive movements occur on a pseudoperiodic basis, with an average interval of 20 to 40 seconds (range, 5 to 90 seconds) and a duration of 0.5 to 5.0 seconds [see Figure 3]. PLMS appears most commonly in RLS and may also occur in a large number of other medical, neurologic, and sleep disorders and with medication (e.g., tricyclic antidepres-sants, SSRIs, and levodopa). PLMS may occur with or without awakenings. Whether PLMS occurs as a primary condition unassociated with RLS causing repeated awakenings and sleep fragmentation remains controversial. In the current International Classification of Sleep Disorders, periodic limb movement disorder (PLMD) is listed as a separate entity.17 A growing body of evidence suggests that PLMS may not have any clinical significance and is simply a polysomnographic observation noted in a variety of sleep disorders but present in the majority of patients with RLS.3536
Treatment
Four groups of drugs are available to treat RLS and PLMS33: dopaminergic drugs (e.g., carbidopa-levodopa and dopamine agonists such as pergolide, pramipexole, ropinirole, and cabergo-line); benzodiazepines (e.g., clonazepam); opioids (e.g., codeine,propoxyphene, oxycodone, and hydrocodone); and anticonvul-sants (e.g., gabapentin). The best drug for initial therapy in most cases is a dopamine agonist. The only drug currently approved by the Food and Drug Administration for use in RLS is ropini-role, but several clinical trials have proved that other agents can be used for this disorder.
Circadian rhythm sleep disorders
In circadian rhythm sleep disorders, patients have difficulty sleeping as a result of desynchronization between their internal circadian rhythms and external time.37 The insomnia of these disorders is often associated with other somatic complaints. The most common circadian rhythm sleep disorders are jet lag (associated with high-speed air travel across time zones) and shiftwork sleep disorder (seen in patients who work nonstandard shifts). In delayed-sleep-phase syndrome, the patient has difficulty sleeping early in the evening, goes to sleep late (e.g., 2 A.M.), and wakes up late in the morning. Patients with advanced-sleep-phase syndrome go to sleep early in the evening and wake up early in the morning; this disorder is often seen in patients with depression and in normal elderly persons.
Appropriately timed bright-light therapy and chronotherapy may be effective in these disorders.37 Jet lag and shift-work sleep disorder may also be treated with zolpidem. Melatonin has been found to be useful in some persons with jet lag and shift-work sleep disorders, as well as in patients with non-24-hour circadi-an rhythm disorders. There is no generally accepted standard dose for melatonin; in general, 0.5 to 3 mg has been used for jet lag, and 5 to 10 mg for non-24-hour syndrome. Melatonin has also been used in combination with bright-light therapy in patients with delayed-sleep-phase syndrome.
Insomnias
Insomnia may occur at any age. The patient may complain of difficulty initiating or maintaining sleep or of awakening early in the morning and being unable to go back to sleep, as well as non-restorative sleep or poor quality of sleep.38 Insomnia may be associated with a variety of medical, psychiatric, and neurologic illnesses or may be drug- or alcohol-induced [see Table 3]. Insomnia is most commonly caused by psychiatric or psychophysio-logic disorders, with depression and anxiety among the most important. Early morning awakening is a characteristic finding in depression. In some cases, no cause of the insomnia is found; this disorder is termed idiopathic, or primary, insomnia and is a lifelong condition.
For transient insomnia or insomnia of short duration, treatment with sedative-hypnotics (e.g., Zolpidem, zaleplon, or eszopi-clone) or short- or intermediate-acting benzodiazepines (e.g., temazepam) for a few nights to a few weeks is appropriate [see Table 4]. Hypnotic medications should not be used for chronic insomnia. The best treatment for patients with chronic insomnia consists of a combination of sleep hygiene measures (e.g., setting fixed times for retiring and awakening; avoidance of caffeinated beverages, tobacco, and alcohol before retiring; and regular exercise, preferably undertaken 4 to 6 hours before bedtime), stimulus-control therapy, sleep restriction, relaxation training, and other psychological treatments.38 One study showed a trend toward better outcomes with a combination of cognitive-behavioral therapy (i.e., stimulus control, sleep restriction, sleep hygiene, and cognitive therapy) and pharmacotherapy.39 Sedative-antidepres-sants should be used for insomnia associated with depression.
Table 4 Short-Term Drug Therapy for Insomnia
|
Category |
Agent |
Dose (mg)* |
|
Sedative-hypnotics |
Zaleplon |
5-10 |
|
Zolpidem |
5-10 |
|
|
Zolpidem extended release |
6.25-12.5 |
|
|
Eszopiclone |
1-3 |
|
|
Ramelteon |
8 |
|
|
Short-acting or intermediate-acting benzodiazepines |
Temazepam |
7.5-30 |
|
Triazolam |
0.125-0.250 |
|
|
Flurazepam |
15-30 |
|
|
Estazolam |
1-2 |
*All agents are given at bedtime for a few nights to a few weeks.
Parasomnias
Partial Arousal Disorders
Partial arousal disorders include confusional arousals, sleepwalking, and sleep terrors.40 Confusional arousals, which occur during slow wave sleep, mostly in children younger than 5 years, are characterized clinically by confusion and mild automatic and inappropriate behavior. Most confusional arousals are benign.
Sleepwalking, or somnambulism, is most common in children between 5 and 12 years of age. Sleepwalking begins with abrupt motor activity arising out of slow wave sleep during the first third of the sleep period. Episodes generally last less than 10 minutes. There is a high incidence of positive family history in sleepwalking. Injuries and violent actions have been reported during sleepwalking episodes. Sleep deprivation, fatigue, concurrent illness, and sedatives may act as precipitating factors.
Sleep terrors, or pavor nocturnus, also occur during slow wave sleep. Peak onset is between 5 and 7 years of age. As with sleepwalking, a high incidence of familial cases is seen in sleep terrors. Episodes of sleep terrors are characterized by intense au-tonomic and motor symptoms, including a loud, piercing scream. Many patients also have sleepwalking episodes. Precipitating factors are similar to those described with sleepwalking.
No special treatment is needed for most of the parasomnias. If attacks of sleepwalking or sleep terrors are frequent, treatment with an antidepressant or benzodiazepine may be tried for a short period.
Nocturnal Frontal Lobe Epilepsy
Nocturnal frontal lobe epilepsy (NFLE) was formerly known as nocturnal paroxysmal dystonia. NFLE may occur at any time from infancy to the fifth decade of life41; the disorder is seldom familial. The attacks occur suddenly during NREM sleep. The most common type is a spell of short duration (lasting from 15 seconds to less than 2 minutes). In rare cases, the spell is of longer duration (lasting from 2 minutes to more than 1 hour). The attacks are characterized by ballismic, choreoathetoid, or dystonic movements, which may occur in clusters. The EEG is generally normal. Carbamazepine is the treatment of choice for NFLE. Several families with autosomal dominant NFLE have been described.42
REM Sleep Behavior Disorder
REM sleep behavior disorder is an important REM sleep parasomnia commonly seen in elderly persons.43 A characteristic clinical feature of RBD is intermittent loss of REM-related muscle hypotonia or atonia and the appearance of a variety of abnormal motor activities during sleep. The patient presents with violent, dream-enacting behavior during REM sleep, often causing self-injury or injury to the patient’s bed partner. Initially, it was thought that RBD was mostly idiopathic, but as more patients were described, it was realized that most cases are secondary and associated with neurodegenerative diseases. RBD occurs with increasing frequency in patients with Parkinson disease (PD), multiple system atrophy (MSA), diffuse Lewy body disease (DLBD), olivopontocerebellar atrophy (OPCA), progressive supranuclear palsy (PSP), and corticobasal ganglionic degeneration (CBD).44 RBD has also been described in many cases of narcolepsy, which may be considered a degenerative disease of hypocretin-containing neurons in the hypothalamus. In many of these neurodegenerative diseases (e.g., PD, MSA, and DLBD), a-synuclein inclusions have been noted; some authors even propose that RBD may be an a-synucleinopathy disorder.44 The findings of reduced striatal presynaptic dopamine transporter on positron emission tomographic scans and of a reduction of postsynaptic D2 dopamine receptors on single-photon emission computed tomography scans suggest the linking of RBD to dopamine cell dysfunction.45 RBD may precede many of these neurodegenerative diseases or coexist with these disorders. RBD may also be associated with structural lesions of the brain stem and is sometimes associated with alcoholism or the ingestion of drugs (e.g., sedative-hypnotics, tricyclic antidepressants, SSRIs, and anticholinergics). On polysomnographic studies, the prominent finding is REM sleep without muscle atonia. In experiments on cats, similar behavior has been produced by a bilateral perilo-cus coeruleus lesion. Most cases of RBD respond to a low dose of a benzodiazepine (e.g., clonazepam).