B cell subsets
Follicular Mantle Cells
Follicular mantle cells derive their name from their location, surrounding the active germinal centers in lymph nodes. These cells express IgD but not CD38 on their surface (i.e., they are IgD+,CD38-) and can mature into plasma cells that produce IgM antibodies only. These cells produce IgM antibodies that display extensive heterogeneity, but the cells do not undergo somatic mutation to generate larger numbers of heterogeneous antibody molecules.
Centrocytes
Centrocytes are found in the germinal center of lymph nodes. The process leading to somatic mutation occurs in the centro-cytes, which are IgD-,CD38+ B cells that have undergone class switching to produce IgG. The maturation step from IgD+,CD38-B cells to IgD-,CD38+ B cells depends on cell-cell contact with antigen-specific T cells and cytokines. IL-4 in particular is of great importance for the events that lead to class switching.
Memory B Cells
The most mature B cell, the memory cell, does not express IgD or CD38 but is distinguished by the presence of other cell surface markers and by its location within the germinal center of lymph nodes. Memory cells can also develop into plasma cells producing IgM, IgG, IgA, and IgE. In general, the generation of plasma cells from memory B cells is independent of T cell help.
T Cell-B Cell Interactions
An important pathway for B cell activation involves CD40, a B cell surface receptor belonging to the TNFR family. The natural ligand for CD40, CD40L (also called CD154), is a glycoprotein related to TNF. Transient CD40L expression on the surface of T cells is induced by the binding of TCR to antigen-MHC complex and by binding of B7 to CD28 or CTLA-4. The binding of CD40 to CD40L stimulates B cell immunoglobulin class switching.
This was best demonstrated by elucidation of the molecular defect in a genetic immunodeficiency termed hyper-IgM syndrome [see 6:VIII Deficiencies in Immunoglobulins and Cell-Mediated Immunity]. Patients with the X-linked form of this syndrome have a mutation in the gene encoding CD40L that results in defective antibody class switching. T cell-independent B cell responses and responses induced by anti-CD40 are unaffected by this mutation. Thus, T cell help for B cell activation is primarily directed through the CD40-CD40L costimulatory pathway. The observation that persons with hyper-IgM syndrome are susceptible to opportunistic infections such as those seen in AIDS— that is, infections from pathogens normally dealt with by T cells—underscores the role of CD40L costimulation in normal T cell activation. Absence of CD40L has a more dramatic effect on immunoglobulin class switching than does an absence of T cells. Thus, it seems that CD40L may be expressed on cells other than T cells that could induce immunoglobulin class switching in B cells through the ligation of CD40.
Cytokines
Cytokines and t cell subsets
Cytokines, a diverse group of proteins produced by a number of different cell types, are critical in the regulation of immune responses [see Table 2]. They are also important in the differentiation of cell systems. In general, cytokines are synthesized in response to stimulation of cells. In some cases, the active cytokine is released from an inactive precursor by prote-olysis. Occasionally, cytokines are stored in cells.
After secretion, cytokines usually act locally by binding to receptors on cell surfaces. Cytokines may act on many different cells, and different cytokines may have similar activities. Cy-tokine receptors are often composed of the same protein chains. For instance, the cytokine receptors IL-2R, IL-4R, IL-7R, IL-9R, IL-13R, and IL-15R have the y chain in common. Most cy-tokines form a network that regulates the activation of cells and the production of other cytokines.17 The binding of cytokines to receptors on cell surfaces activates intracellular signaling mechanisms, which lead to expression of particular genes (e.g., genes for the bound cytokine, or other cytokines). The principal intra-cellular cytokine signal transducers are two families of transcription factors: Jak (Janus kinase) protein tyrosine kinases and STAT (signal transducers and activators of transcription).
Table 1 Costimulatory Molecules on T Cells
|
Molecule |
Ligand |
Function |
|
CD4 |
MHC molecules on the surface of APCs |
Helps generate CD4+ T cells in the thymus; directly influences TCR-initiated signaling |
|
CD8 |
MHC molecules on the surface of APCs |
Helps generate CD8+ T cells in the thymus; directly influences TCR-initiated signaling |
|
CD2 |
CD58 on the surface of APCs |
Promotes T cell activation and proliferation |
|
CD11/CD18 |
ICAM-1 (CD54), the complement component iC3b, extracellular matrix proteins |
Promotes T cell activation and proliferation |
|
CD28 |
B7-1 (CD80), B7-2 (CD86) on the surface of APCs |
Promotes T cell activation and proliferation |
|
CTLA-4 |
B7-1 (CD80), B7-2 (CD86) on the surface of APCs |
Inhibits T cell activation and proliferation |
|
SLAM* |
SLAM receptors on the surface of APCs |
Promotes activation and proliferation of T cells and other immune cells |
|
CD27 |
CD70 on activated T and B cells |
T cell proliferation |
|
Fas (APO-1; CD95) |
CD95L on cytolytic effector cells |
Causes apoptosis or promotes TCR-CD3 activation |
*Also found on natural killer cells.
APC—antigen-presenting cell
CTLA-4—cytotoxic T lymphocyte-associated antigen-4
ICAM-1—intercellular adhesion molecule-1
SLAM—signaling lymphocyte activation molecule
TCR—T cell receptor
Figure 3 The downstream signaling pathways induced after TCR stimulation are shown. Phosphorylation of the cytoplasmic tails of the CD3-y, CD3-6, CD3-e, and CD3-| by the Src kinase Fyn or Lck recruits the kinase Syk or ZAP-70, which relays the signal of TCR binding through tyrosine phosphorylation of two adapter proteins, LAT and SLP-76. Nck is an adapter protein associated with SLP-76. These adapter proteins act on components of classic signal transduction pathways, including phospholipase C-y1 (PLC-y1), growth factor receptor-bound protein-2 (Grb-2, a protein-linking receptor tyrosine kinase), the p85 subunit of phosphatidylinositol-3-kinase (PI-3 kinase), and possibly Vav (a guanosine triphosphate/guanosine diphosphate [GTP/GDP] exchange factor for Rho-family GTPases, including Ras and Rac). Activation of PLC-y causes the release of diacylglycerol, which in turn activates protein kinase C (PKC). PKC is involved in initiating the cascade of other serine-threonine kinases, including Raf-1, mitogen-activated protein (MAP) kinase, and MAP kinase kinase (MEK). The central signal transduction molecule Ras appears to be involved in late events after PKC activation. The Ras and Rac pathways and the serine-threonine kinase pathways are coupled; they activate early genes, such as jun and fos. The Ca2+ generated by PLC-y1 activates another downstream cascade, the calcineurin pathway. Calcineurin, a serine-threonine phosphatase, is a calcium- and calmodulin-dependent enzyme involved in induction of transcription factor NFAT (nuclear factor for activated T cells). PI-3 kinase is a ubiquitous enzyme in the mitogenic signaling and apoptotic pathways of both receptor and nonreceptor protein tyrosine kinases. These various signaling pathways converge by delivering a distinct set of transcription factors, including Jun, Elk, NFAT, Fos, and AP-1 (all DNA-binding proteins), to the promoter region of the IL-2 gene, stimulating expression of the gene and production of IL-2.
Helper T cells
Cytokines play a major role in T cell development and regulation. Indeed, the two helper T cell subsets, Th1 and Th2, are defined by the cytokines they produce [see 6:X Allergic Response]. Th1 and Th2 cells play key roles in determining the balance between host resistance and immunopathology.18 Th1 responses can help eradicate infectious agents, but a Th1-domi-nated response that is poorly effective or too prolonged can result in host damage. Th2 responses are primarily involved in allergic reactions, antibody production, and antibody class switching. They can limit potentially harmful Th1-mediated responses and may be part of the suppressor mechanism for exaggerated or inappropriate Th1 responses.19
Each of the two helper T cell subsets inhibits the development and function of the other. Interferon gamma (IFN-y) produced by Th1 cells inhibits the development and function of Th2 cells, whereas IL-4 and IL-10 produced by Th2 cells inhibit the development and function of Th1 cells. IL-4 acts partly by downregulating the expression of the IL-12 receptor, IL-12Rp, which is upregulated by IFN-y [see Figure 4]. IL-12 enhances cell function because it is a potent growth factor for NK cells, which also produce IFN-y. Presumably, the reason Th1 and Th2 cells inhibit each other is that both subsets also induce inflammation, which must be regulated [see Inflammatory Cytokines and Anti-inflammatory Cytokines, below].
Table 2 Selected Cytokines
|
Cytokine |
Sources |
Principal Functions |
Comments |
|
Interferon-y (IFN-y) |
T cells, NK cells |
Primary macrophage-activating factor (MAF) |
Used to treat chronic granulomatous disease, drug-resistant leishmaniasis |
|
Interleukin-1 |
Monocytes, macrophages, other cells |
MAF, endogenous pyrogen |
IL-1 inhibitors counteract rheumatoid arthritis and other inflammatory processes |
|
Interleukin-2 |
Th1 cells |
Activation of lymphocytes, NK cells; MAF |
Used in cancer therapy |
|
Interleukin-4 |
Th1 cells, other cells |
Activation of lymphocytes, monocytes; B cell class switching |
— |
|
Interleukin-5 |
Th2 cells, activated mast cells |
Eosinophil recruitment and activation |
— |
|
Interleukin-6 |
Th1 cells, macrophages |
Activates T cells and macrophages, promotes inflammation |
— |
|
Interleukin-8 |
T cells, macrophages |
Activates neutrophils |
— |
|
Interleukin-10 |
T cells, B cells, macrophages, activated mast cells, keratinocytes |
Suppresses lymphocyte responses by downregu-lating macrophage cytokines |
— |
|
Interleukin-12 |
Macrophages |
Stimulates development of Th1 cells, stimulates production of IFN-y by Th1 and NK cells |
Possible use as vaccine adjuvant |
|
Interleukin-18 |
Macrophages |
Stimulates production of IFN-y |
— |
|
Lymphotoxin |
Th1 cells |
Lyses tumor cells, activates neutrophils, increases vascular adhesion and extravasation of leukocytes |
— |
|
Migration inhibitory factor |
Macrophages, T cells |
Counterregulates glucocorticoid action |
— |
|
Transforming growth factor-p |
Platelets, lymphocytes, activated macro-phages, placenta cells, others |
Anti-inflammatory; inhibits activation of macrophages and maturation of cytotoxic T cells |
— |
|
Tumor necrosis factor-a |
Macrophages, activated T cells, NK cells, mast cells |
Enhances protective inflammatory response |
Antibodies to TNF-a are used in the treatment of Crohn disease and rheumatoid arthritis |
NK—natural killer
Th1—type 1 helper
T cells
Th2—type 2 helper T cells
Certain microorganisms may affect the relative balance of Th1 and Th2. For example, the parasite Schistosoma drives a strong Th2 immune response. A carbohydrate found on the surface of Schistosoma, lacto-N-fucopentaose-III (LNFP-III), induces expansion of B1 cells and secretion of large amounts of IL-10. Acting through macrophages, IL-10 both blocks development of Th1 cells and favors Th2 responses.20,21 A related carbohydrate, lacto-N-neotetraose (LNnT)—which is found on other helminths, Helicobacter pylori, Neisseria meningitidis, and other microorganisms—has a mode of action similar to that of LNFP-III.22 LNFP-III is also found on the surface of cancer cells, and LNnT is found in human milk, which suggests that in these circumstances, as well, the two carbohydrates are being used to shut off the host’s protective cell-mediated immune response. Logically, such carbohydrates may have therapeutic value for the inhibition of Th1-initiated autoimmune diseases.
Memory t cells
On reexposure to an antigen, memory cells mediate a faster and stronger immune response than naive T cells. Memory cells secrete a full range of cytokines and may show the same selection of cytokines as Th1 and Th2 cells.10 The requirements for proliferation and cytokine production in memory cells are not as strict as those for production in naive T cells, but optimum responses require costimulation.23 Memory T cells selectively migrate (home) to specific nonlymphoid tissues such as gut, skin, and lung. Those that home to the gut have specialized integrins (adhesion molecules) on their surface that mediate this migration [see 6:I Organs and Cells of the Immune System]. In the absence of antigenic stimulation, memory cells appear to persist as nondividing cells. A reencounter with an antigen can expand the population to a stable, higher level; competition from another antigen can decrease the population.
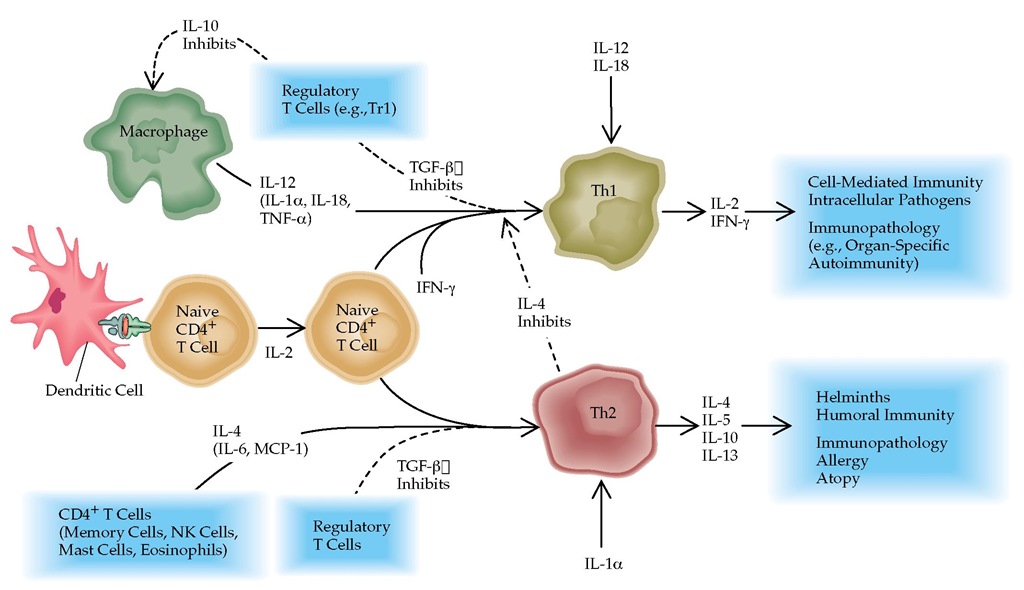
Figure 4 Regulation of helper T cell responses. In response to IL-12 and cofactors such as IL-18 and IL-1a, naive CD4+ T cells can develop into type 1 helper T (Th1) cells responsible for cell-mediated immunity; differentiation is dependent on interferon gamma (IFN-y). Th1 cells produce IFN-y and IL-2. Th1 responses are directly antagonized by IL-4 and indirectly by IL-10, as it inhibits production of IL-12 and IL-18 by macrophages. Th2 cells, which are responsible for inducing antibody production by B cells and allergic responses, depend on IL-4 for differentiation from naive CD4+ T cells. Th2 cells produce IL-4, IL-5, IL-10, and IL-13. TGF-| can inhibit both Th1 and Th2 development. (MCP—monocyte chemoattractant protein; TGF—transforming growth factor)
Regulatory t cells
Natural regulatory T (Treg) cells are a population of T cells that primarily regulate responses to self antigens by other T cells and, therefore, contribute to the maintenance of self-tolerance. For example, removal of certain Treg cells from the periphery of normal mice leads to spontaneous development of various autoimmune and inflammatory diseases (e.g., autoimmune thyroiditis, gastritis, type 1 diabetes mellitus, and inflammatory bowel disease). Treg cells function via secreted or membrane-bound transforming growth factor-| (TGF-|) and by secretion of IL-10.
Most Treg cells are CD4+ and constitutively express CD25 on their surface. CD4+,CD25+ T cells are produced by the normal thymus as a functionally mature T cell subpopulation with a broad T cell repertoire; they can recognize both self and nonself antigens.25 The Foxp-3 gene serves as a marker of this natural regulatory population. However, induction of Treg cells also takes place in the periphery (adaptive Treg cells).26 Chronic activation of human CD4+ T cells in the presence of IL-10 gives rise to a subset of antigen-specific CD4+ T cell clones with low proliferative capacity that produce high levels of IL-10, low levels of IL-2, and no IL-4. These T cells, called type 1 regulatory T (Tr1) cells, suppress the proliferation of CD4+ T cells in response to antigen, which suppresses antigen-specific immune responses and actively downregulates a pathologic immune response.
![The downstream signaling pathways induced after TCR stimulation are shown. Phosphorylation of the cytoplasmic tails of the CD3-y, CD3-6, CD3-e, and CD3-| by the Src kinase Fyn or Lck recruits the kinase Syk or ZAP-70, which relays the signal of TCR binding through tyrosine phosphorylation of two adapter proteins, LAT and SLP-76. Nck is an adapter protein associated with SLP-76. These adapter proteins act on components of classic signal transduction pathways, including phospholipase C-y1 (PLC-y1), growth factor receptor-bound protein-2 (Grb-2, a protein-linking receptor tyrosine kinase), the p85 subunit of phosphatidylinositol-3-kinase (PI-3 kinase), and possibly Vav (a guanosine triphosphate/guanosine diphosphate [GTP/GDP] exchange factor for Rho-family GTPases, including Ras and Rac). Activation of PLC-y causes the release of diacylglycerol, which in turn activates protein kinase C (PKC). PKC is involved in initiating the cascade of other serine-threonine kinases, including Raf-1, mitogen-activated protein (MAP) kinase, and MAP kinase kinase (MEK). The central signal transduction molecule Ras appears to be involved in late events after PKC activation. The Ras and Rac pathways and the serine-threonine kinase pathways are coupled; they activate early genes, such as jun and fos. The Ca2+ generated by PLC-y1 activates another downstream cascade, the calcineurin pathway. Calcineurin, a serine-threonine phosphatase, is a calcium- and calmodulin-dependent enzyme involved in induction of transcription factor NFAT (nuclear factor for activated T cells). PI-3 kinase is a ubiquitous enzyme in the mitogenic signaling and apoptotic pathways of both receptor and nonreceptor protein tyrosine kinases. These various signaling pathways converge by delivering a distinct set of transcription factors, including Jun, Elk, NFAT, Fos, and AP-1 (all DNA-binding proteins), to the promoter region of the IL-2 gene, stimulating expression of the gene and production of IL-2. The downstream signaling pathways induced after TCR stimulation are shown. Phosphorylation of the cytoplasmic tails of the CD3-y, CD3-6, CD3-e, and CD3-| by the Src kinase Fyn or Lck recruits the kinase Syk or ZAP-70, which relays the signal of TCR binding through tyrosine phosphorylation of two adapter proteins, LAT and SLP-76. Nck is an adapter protein associated with SLP-76. These adapter proteins act on components of classic signal transduction pathways, including phospholipase C-y1 (PLC-y1), growth factor receptor-bound protein-2 (Grb-2, a protein-linking receptor tyrosine kinase), the p85 subunit of phosphatidylinositol-3-kinase (PI-3 kinase), and possibly Vav (a guanosine triphosphate/guanosine diphosphate [GTP/GDP] exchange factor for Rho-family GTPases, including Ras and Rac). Activation of PLC-y causes the release of diacylglycerol, which in turn activates protein kinase C (PKC). PKC is involved in initiating the cascade of other serine-threonine kinases, including Raf-1, mitogen-activated protein (MAP) kinase, and MAP kinase kinase (MEK). The central signal transduction molecule Ras appears to be involved in late events after PKC activation. The Ras and Rac pathways and the serine-threonine kinase pathways are coupled; they activate early genes, such as jun and fos. The Ca2+ generated by PLC-y1 activates another downstream cascade, the calcineurin pathway. Calcineurin, a serine-threonine phosphatase, is a calcium- and calmodulin-dependent enzyme involved in induction of transcription factor NFAT (nuclear factor for activated T cells). PI-3 kinase is a ubiquitous enzyme in the mitogenic signaling and apoptotic pathways of both receptor and nonreceptor protein tyrosine kinases. These various signaling pathways converge by delivering a distinct set of transcription factors, including Jun, Elk, NFAT, Fos, and AP-1 (all DNA-binding proteins), to the promoter region of the IL-2 gene, stimulating expression of the gene and production of IL-2.](http://what-when-how.com/wp-content/uploads/2012/04/tmp4C5_thumb.jpg)